Answered step by step
Verified Expert Solution
Question
1 Approved Answer
SOLVE THE LAST PART ONLY PLEASE. THE OTHER PARTS ARE CORRECT SO BASE ANY WORK FOR THE LAST PART OFF OF THOSE. THANK YOU. Please
SOLVE THE LAST PART ONLY PLEASE. THE OTHER PARTS ARE CORRECT SO BASE ANY WORK FOR THE LAST PART OFF OF THOSE. THANK YOU.
Please use my data for it and not another problems as well. Thanks.
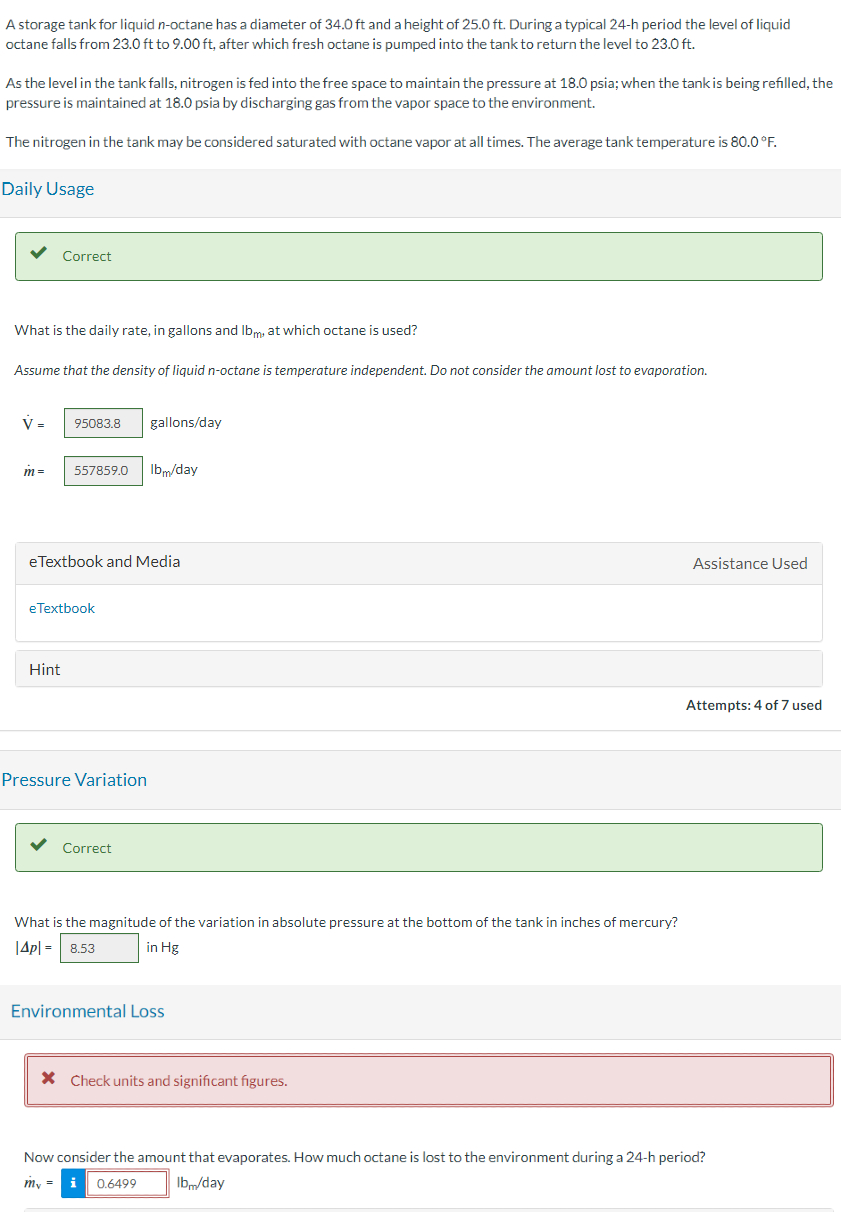
A storage tank for liquid octane has a diameter of and a height of During a typical period the level of liquid
octane falls from to after which fresh octane is pumped into the tank to return the level to
As the level in the tank falls, nitrogen is fed into the free space to maintain the pressure at psia; when the tank is being refilled, the
pressure is maintained at psia by discharging gas from the vapor space to the environment.
The nitrogen in the tank may be considered saturated with octane vapor at all times. The average tank temperature is
Daily Usage
Correct
Pressure Variation
Correct
Environmental Loss
Now consider the amount that evaporates. How much octane is lost to the environment during a h period? lbmday
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


