Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve the problem using matlab code It turns out that Equations 1 and 2 above can be solved analytically to get the following solution for

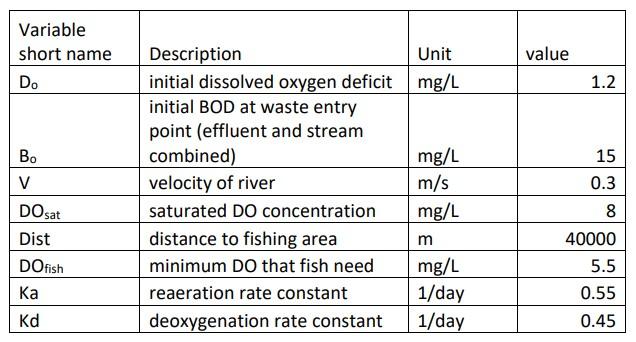
Solve the problem using matlab code
It turns out that Equations 1 and 2 above can be solved analytically to get the following solution for the oxygen deficit changing with time: D(t)=KaKdKdBo(eKdteKat)+DoeKat (in this equation, the exponential terms should be Kdt and Kat, where a and d are subscripts) a) Using Eq. 3, solve for D, and then DO=DOsatD over time. Plot these values versus time for several days. b) Next, we need to know the conditions at the location of the fishing hole. How long does water take to reach this location (hint: you are given the speed of the flow and the distance). Now, plot D and DO with distance instead of time, and mark the point of the fishing hole. c) How far downstream does the DO rise above 5.5 ? how about back to its initial concentration of 7mg/L ? d) Is there a problem for the fish survival at this location? \begin{tabular}{|l|l|l|r|} \hline Variableshortname & Description & Unit & value \\ \hline Do & initial dissolved oxygen deficit & mg/L & 1.2 \\ \hline & initialBODatwasteentrypoint(effluentandstreamcombined) & & 15 \\ \hline Bo & velocity of river & mg/L & 0.3 \\ \hline DO sat & saturated DO concentration & mg/L & 8 \\ \hline Dist & distance to fishing area & m & 40000 \\ \hline DO fish & minimum DO that fish need & mg/L & 5.5 \\ \hline Ka & reaeration rate constant & 1/day & 0.55 \\ \hline Kd & deoxygenation rate constant & 1/day & 0.45 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


