Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve this equation by Simpson and make table and graph A homogeneous gas reaction A3R has a reported rate at 215C rA=102CA1/2,[mol/litersec] Find the space-time
solve this equation by Simpson and make table and graph 
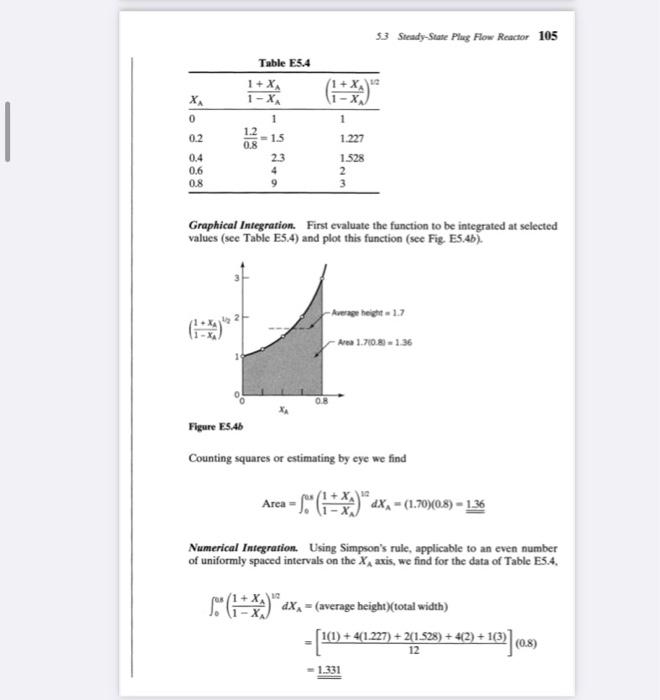
A homogeneous gas reaction A3R has a reported rate at 215C rA=102CA1/2,[mol/litersec] Find the space-time needed for 80% conversion of a 50%A50% inert feed to a plug flow reactor operating at 215C and 5 atm (CA0=0.0625mol/ liter). OLUTION For this stoichiometry and with 50% inerts, two volumes of feed gas would give four volumes of completely converted product gas; thus A=242=1 in which case the plug flow performance equation, Eq. 17, becomes =CA00xNrAdXA=CA00xNkCA01/2(1+AXA1XA)1/2dXA=kCA01/000.8(1XA1+XA)1/2dXA The integral can be evaluated in any one of three ways: graphically, numerically, or analytically. Let us illustrate these methods. 5.3 Steady-State Plugg Flow Renctor 105 Graphical Integration. First evaluate the function to be integrated at selected values (see Table E5.4) and plot this function (see Fig. E5.4b). Figure E.5.4b Counting squares or estimating by eye we find Area=0s(1XA1+XA)12dXA=(1.70)(0.8)=1.36 Numerical Integration. Using Simpson's rule, applicable to an even number of uniformly spaced intervals on the XA axis, we find for the data of Table E5.4. 0es(1XA1+XA)1/2dXA=(averagebeight)(totalwidth)=[121(1)+4(1.227)+2(1.528)+4(2)+1(3)](0.8)=1.331 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


