Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve this Question and explained each steps completely. [5] A liquid mixture containing 60 mol% acetone and 40 mol% water at 80F is to be
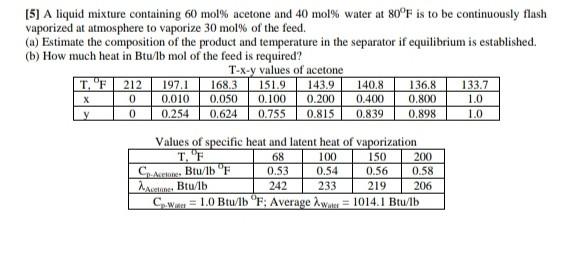
Solve this Question and explained each steps completely.
[5] A liquid mixture containing 60 mol% acetone and 40 mol% water at 80F is to be continuously flash vaporized at atmosphere to vaporize 30 mol% of the feed. (a) Estimate the composition of the product and temperature in the separator if equilibrium is established. (b) How much heat in Btu/lb mol of the feed is required? T-X-y values of acetone TF 212 197.1 168.3 151.9 143.9 140.8 136,8 133.7 0.010 0.050 0.100 0.200 0.400 0.800 1.0 y 0 0.254 0.624 0.755 0.815 0.839 0.898 1.0 X 0 Values of specific heat and latent heat of vaporization 68 100 150 200 C. Btu/lb F 0.53 0.54 0.56 0.58 Act. Btu/lb 242 233 219 206 C.We = 1.0 Btu/lb F; Average wote = 1014.1 Btu/lb SalaStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


