Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve this with graphs and drawings An acetone - water solution containing 2 5 w t % acetone is to be fractionated at a rate
solve this with graphs and drawings
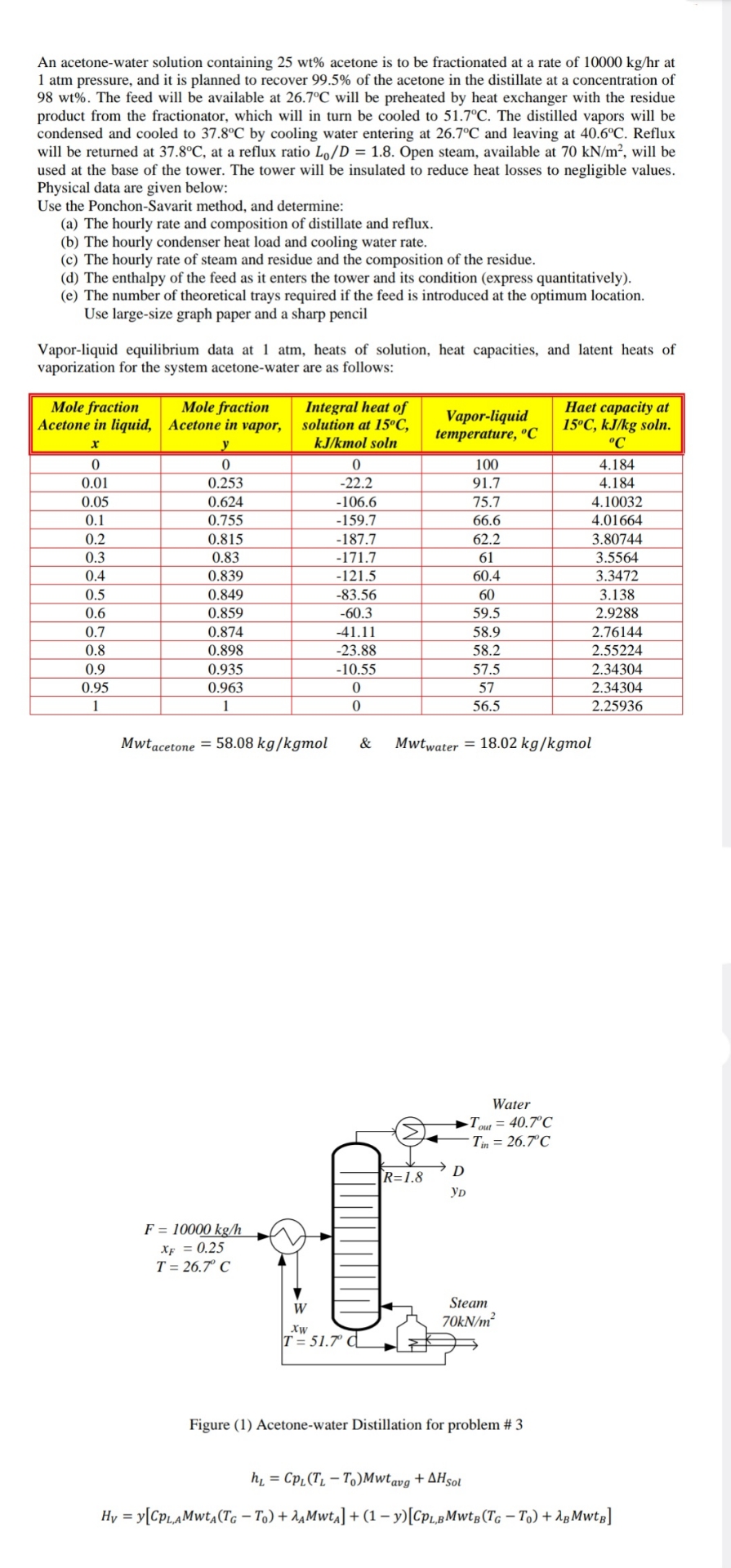
An acetonewater solution containing acetone is to be fractionated at a rate of at atm pressure, and it is planned to recover of the acetone in the distillate at a concentration of The feed will be available at will be preheated by heat exchanger with the residue product from the fractionator, which will in turn be cooled to The distilled vapors will be condensed and cooled to by cooling water entering at and leaving at Reflux will be returned at at a reflux ratio Open steam, available at will be used at the base of the tower. The tower will be insulated to reduce heat losses to negligible values. Physical data are given below:
Use the PonchonSavarit method, and determine:
a The hourly rate and composition of distillate and reflux.
b The hourly condenser heat load and cooling water rate.
c The hourly rate of steam and residue and the composition of the residue.
d The enthalpy of the feed as it enters the tower and its condition express quantitatively
e The number of theoretical trays required if the feed is introduced at the optimum location. Use largesize graph paper and a sharp pencil
Vaporliquid equilibrium data at atm, heats of solution, heat capacities, and latent heats of vaporization for the system acetonewater are as follows:
tabletableMole fractionAcetone in liquid,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


