Answered step by step
Verified Expert Solution
Question
1 Approved Answer
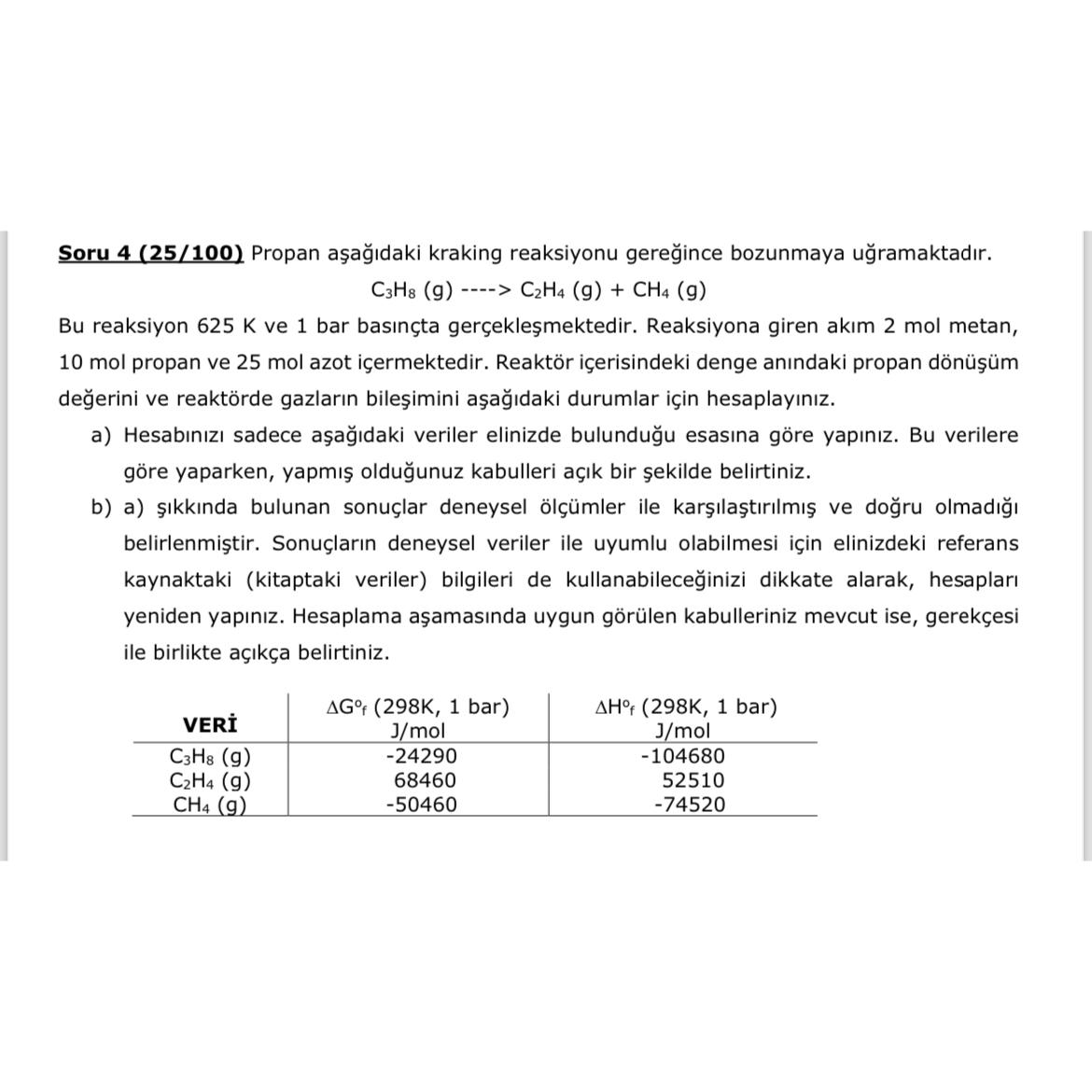
Soru 4 ( 2 5 / 1 0 0 ) Propan a a daki kraking reaksiyonu gere ince bozunmaya u ramaktad r . C 3
Soru Propan aadaki kraking reaksiyonu gereince bozunmaya uramaktadr
cdots
Bu reaksiyon ve bar basnta gereklemektedir Reaksiyona giren akm mol metan, mol propan ve mol azot iermektedir Reaktr ierisindeki denge anndaki propan dnm deerini ve reaktrde gazlarn bileimini aadaki durumlar iin hesaplaynz
a Hesabnz sadece aadaki veriler elinizde bulunduu esasna gre yapnz Bu verilere gre yaparken, yapm olduunuz kabulleri ak bir ekilde belirtiniz.
b akknda bulunan sonular deneysel lmler ile karlatrlm ve doru olmad belirlenmitir Sonularn deneysel veriler ile uyumlu olabilmesi iin elinizdeki referans kaynaktaki kitaptaki veriler bilgileri de kullanabileceinizi dikkate alarak, hesaplar yeniden yapnz Hesaplama aamasnda uygun grlen kabulleriniz mevcut ise, gerekesi ile birlikte aka belirtiniz.
table bar
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


