Answered step by step
Verified Expert Solution
Question
1 Approved Answer
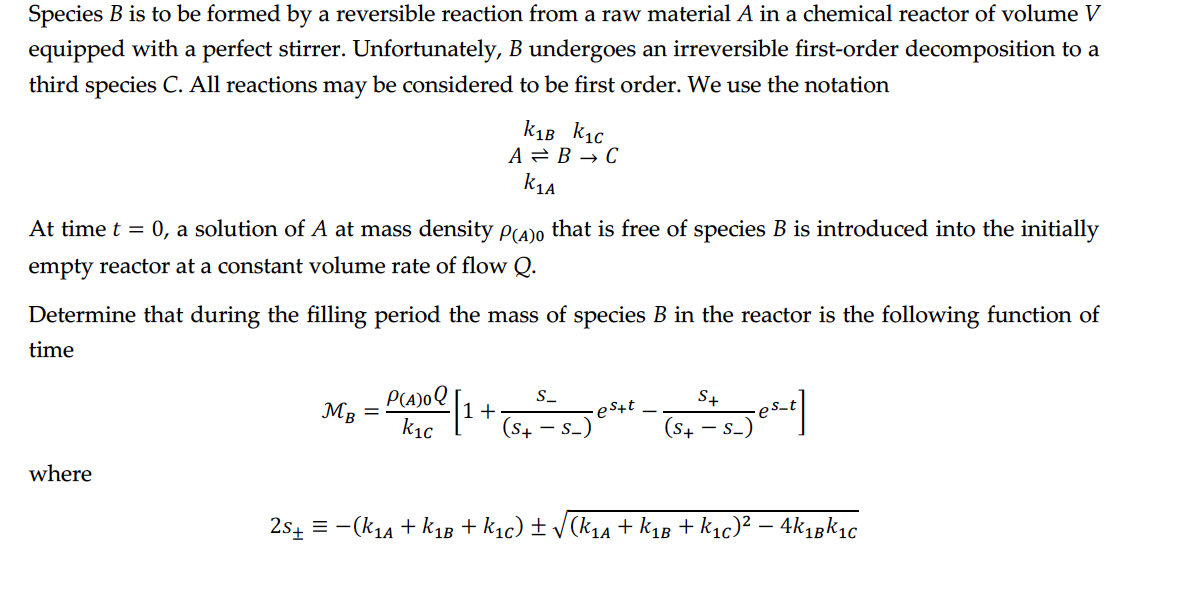
Species B is to be formed by a reversible reaction from a raw material A in a chemical reactor of volume V equipped with a
Species is to be formed by a reversible reaction from a raw material in a chemical reactor of volume
equipped with a perfect stirrer. Unfortunately, undergoes an irreversible firstorder decomposition to a
third species All reactions may be considered to be first order. We use the notation
At time a solution of at mass density that is free of species is introduced into the initially
empty reactor at a constant volume rate of flow
Determine that during the filling period the mass of species in the reactor is the following function of
time
where
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


