Question
Standard potential of S(s)/S system depends on pH of solution. Using the following information: S(s) + 2e S (main system) E =- 0.476 V
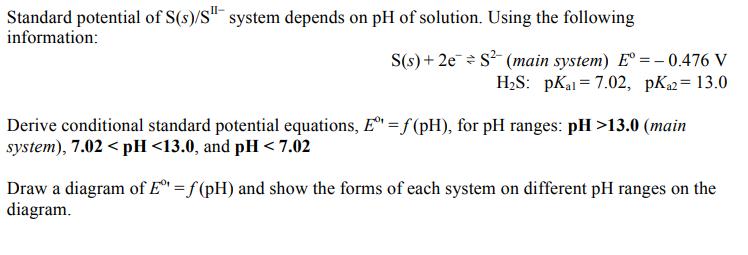
Standard potential of S(s)/S" system depends on pH of solution. Using the following information: S(s) + 2e S (main system) E =- 0.476 V H;S: pKai = 7.02, pK= 13.0 Derive conditional standard potential equations, E = f (pH), for pH ranges: pH >13.0 (main system), 7.02 < pH
Step by Step Solution
3.50 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of Accounting
Authors: Belverd E. Needles, Marian Powers and Susan V. Crosson
12th edition
978-1133603054, 113362698X, 9781285607047, 113360305X, 978-1133626985
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



