Answered step by step
Verified Expert Solution
Question
1 Approved Answer
state assumptions for like 4. P5.48 The oxidation of nitric oxide 25C NO+21O2NO2 takes place in an isothermal batch reactor at 25C. The reactor is
state assumptions for like 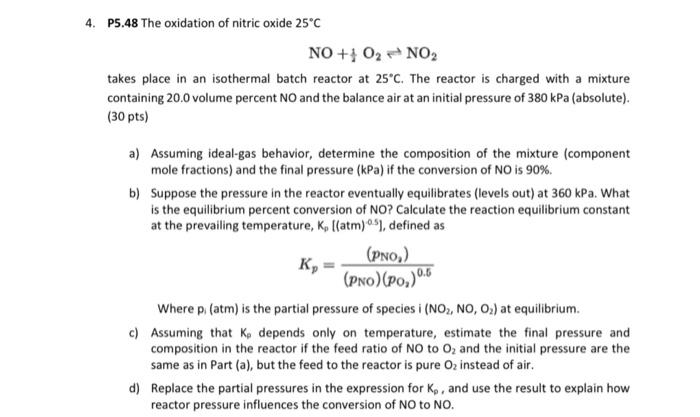
4. P5.48 The oxidation of nitric oxide 25C NO+21O2NO2 takes place in an isothermal batch reactor at 25C. The reactor is charged with a mixture containing 20.0 volume percent NO and the balance air at an initial pressure of 380kPa (absolute). (30 pts) a) Assuming ideal-gas behavior, determine the composition of the mixture (component mole fractions) and the final pressure ( kPa ) if the conversion of NO is 90%. b) Suppose the pressure in the reactor eventually equilibrates (levels out) at 360kPa. What is the equilibrium percent conversion of NO? Calculate the reaction equilibrium constant at the prevailing temperature, Kp[(atm)05], defined as Kp=(pNO)(pO2)0.6(pNO2) Where pi(atm) is the partial pressure of species i (NO2,NO,O2) at equilibrium. c) Assuming that K depends only on temperature, estimate the final pressure and composition in the reactor if the feed ratio of NO to O2 and the initial pressure are the same as in Part (a), but the feed to the reactor is pure O2 instead of air. d) Replace the partial pressures in the expression for Kp, and use the result to explain how reactor pressure influences the conversion of NO to NO 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


