Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Step 1 7 a . Starting with a 0 . 0 5 0 % solution of Red 4 0 dye, show how to calculate the
Step a Starting with a solution of Red dye, show how to calculate the of that solution needed to prepare of dye. Show your work in the space below, as well as the correct answer, with the correct units and to the correct number of significant figures. Then, place your answer in the appropriate cell in the spreadsheet.
Volume of first solution required to make the diluted sample in unknown value
Desired final volume
Percentage concentration
Desired final percentage concentration
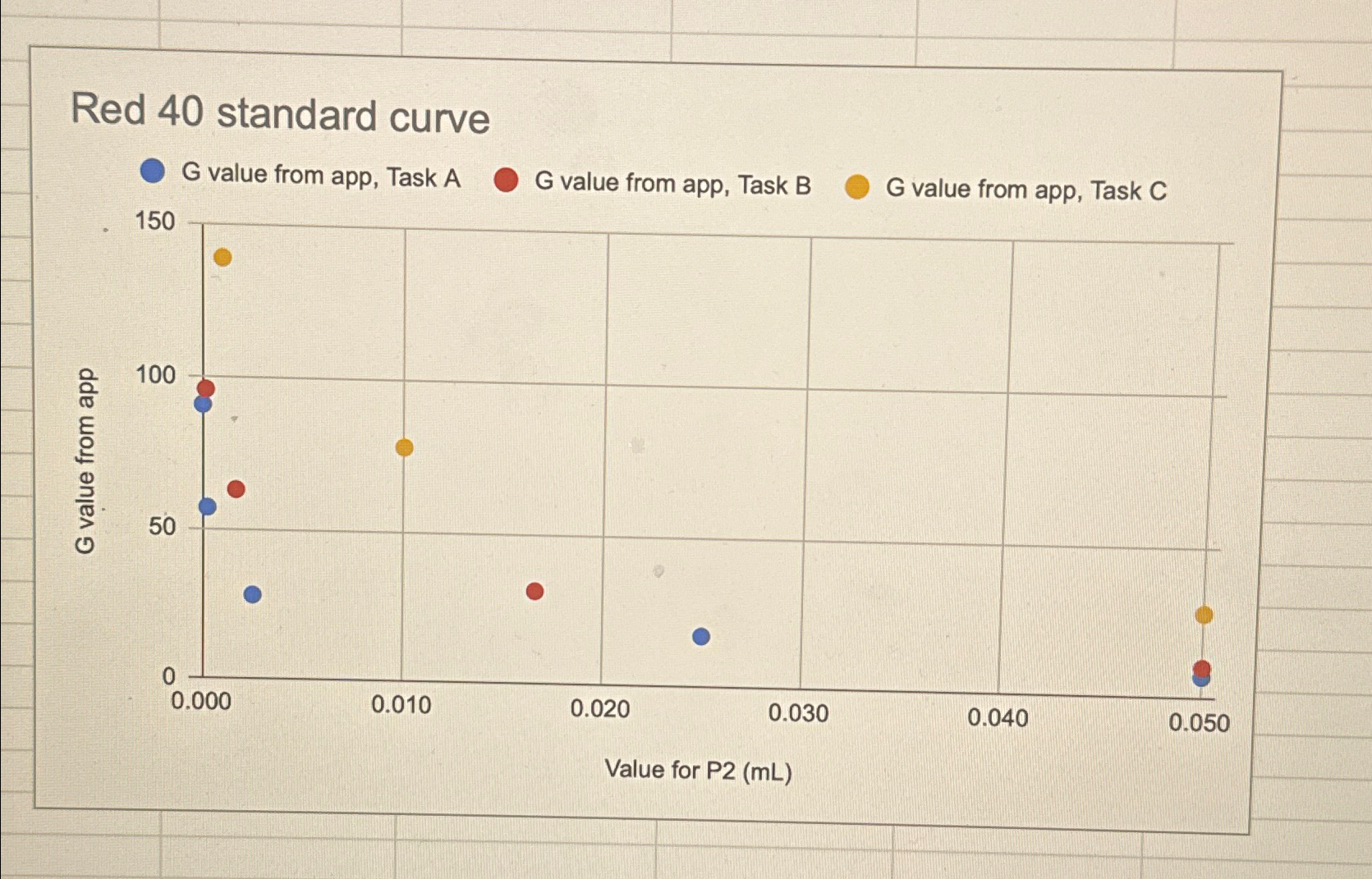
Step b Answer the following question: Using your graph, the value for this dye solution is estimated to be
Red standard curve
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


