Answered step by step
Verified Expert Solution
Question
1 Approved Answer
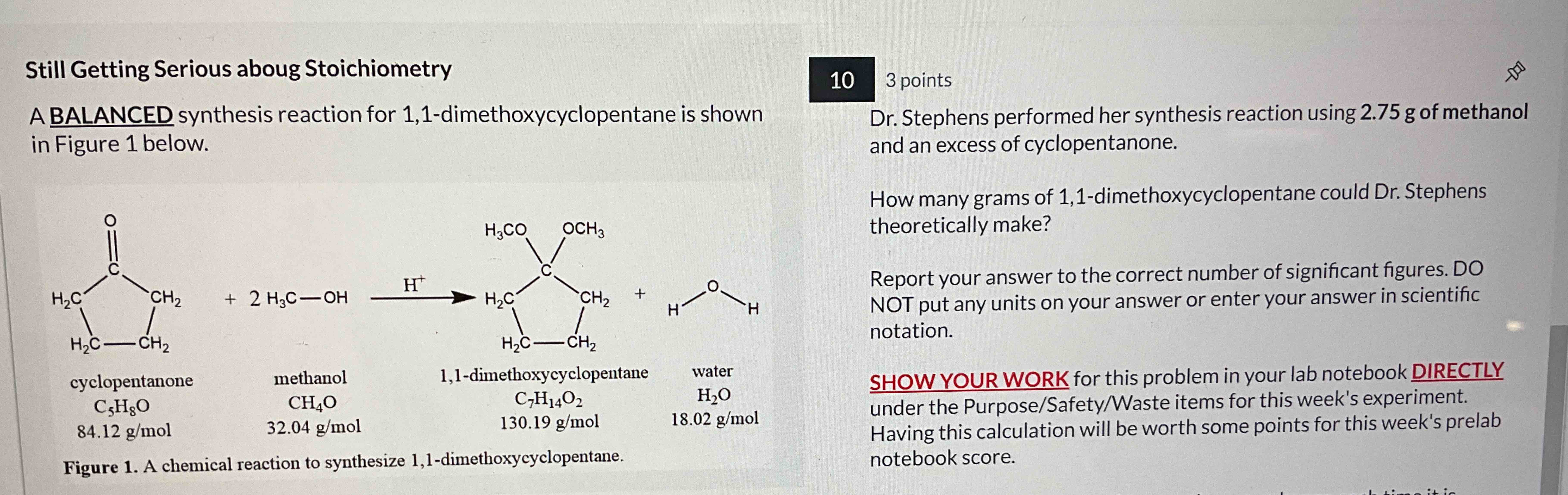
Still Getting Serious aboug StoichiometryStill Getting Serious aboug Stoichiometry A BALANCED synthesis reaction for 1 , 1 - dimethoxycyclopentane is shown in Figure 1 below.
Still Getting Serious aboug StoichiometryStill Getting Serious aboug Stoichiometry
A BALANCED synthesis reaction for dimethoxycyclopentane is shown
in Figure below.
cyclopentanone
methanol
dimethoxycyclopentane
Figure A chemical reaction to synthesize dimethoxycyclopentane.
Dr Stephens performed her synthesis reaction using of methanol
and an excess of cyclopentanone.
How many grams of dimethoxycyclopentane could Dr Stephens
theoretically make?
Report your answer to the correct number of significant figures. DO
NOT put any units on your answer or enter your answer in scientific
notation.
SHOW YOUR WORK for this problem in your lab notebook DIRECTLY
under the PurposeSafetyWaste items for this week's experiment.
Having this calculation will be worth some points for this week's prelab
notebook score.
A BALANCED synthesis reaction for dimethoxycyclopentane is shown
in Figure below.
cyclopentanone
methanol
methanol dimethoxycyclopentane
Figure A chemical reaction to synthesize dimethoxycyclopentane.
Dr DeVries performed her synthesis reaction with of
cyclopentanone.
How many grams of methanol will Dr DeVries need to complete here
reaction?
Report your answer to the correct number of significant figures. DO
NOT put any units on your answer or enter your answer in scientific
notation.
SHOW YOUR WORK for this problem in your lab notebook DIRECTLY
under the PurposeSafetyWaste items for this week's experiment.
Having this calculation will be worth some points for this week's prelab
notebook score.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


