Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Study problem thermodynamics An ideal gas in state A (see the figure) undergoes a reversible change to state C. This transformation can be carried out
Study problem thermodynamics
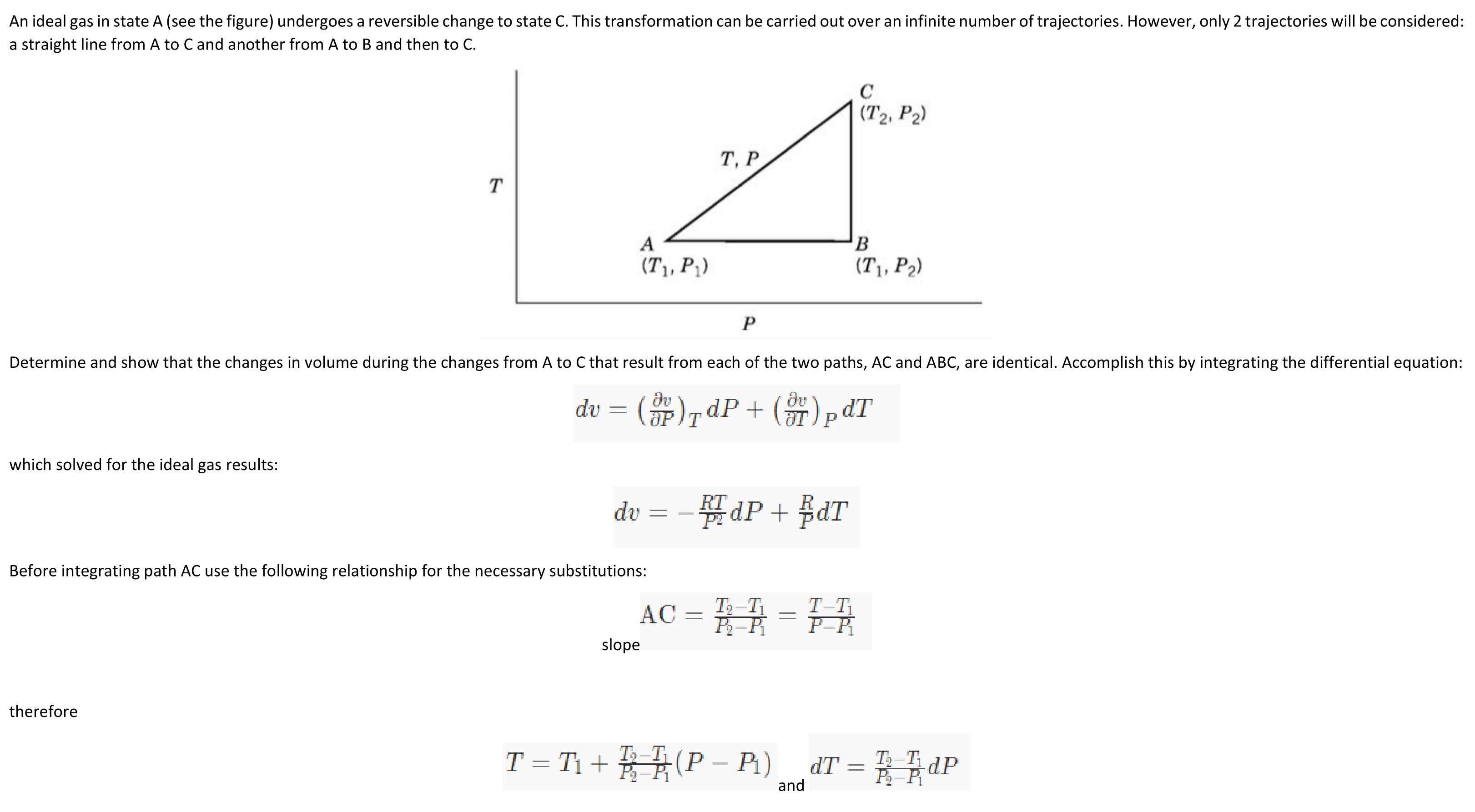
An ideal gas in state A (see the figure) undergoes a reversible change to state C. This transformation can be carried out over an infinite number of trajectories. However, only 2 trajectories will be considered: a straight line from A to C and another from A to B and then to C. Determine and show that the changes in volume during the changes from A to C that result from each of the two paths, AC and ABC, are identical. Accomplish this by integrating the differential equation: (F)TdP + (+) p dT dv which solved for the ideal gas results: dv Before integrating path AC use the following relationship for the necessary substitutions: AC slope therefore 131) TQ TldP and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


