Question
Study the electrode potentials for the half cells below and use them to answer the questions that follow. The letters do not represent the
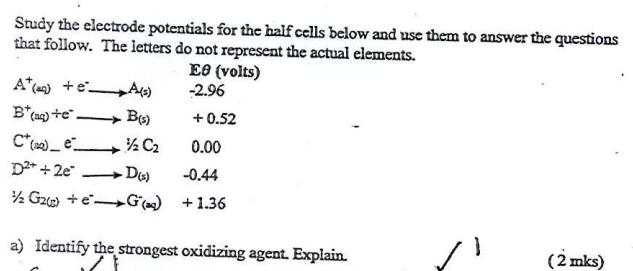



Study the electrode potentials for the half cells below and use them to answer the questions that follow. The letters do not represent the actual elements. A(+eA(=) B*(a)te. C*(a)_e__ D+ + 2e -D(s) 2 G2) +eG() B(s) C EO (volts) -2.96 +0.52 0.00 -0.44 +1.36 a) Identify the strongest oxidizing agent. Explain. (2 mks) b) Which of the two half cells would produce the highest potential difference when combined. / \(2 mks) c) Explain whether the reaction below can take place. D(s) +2A* (ag) D+ (aq) + 2A(s) d) Draw a well labelled diagram when combining A and B half cells. negative. (3 mks)
Step by Step Solution
3.50 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microeconomics
Authors: Paul Krugman, Robin Wells
3rd edition
978-1429283427, 1429283424, 978-1464104213, 1464104212, 978-1429283434
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



