Question
Subject; Thermodynamics Using the book oh Thermodynamics. Cengel, Y.A., and Boles, M.A. (2008). Thermodynamics: An Engineering Approach. 6th ed. McGraw Hill. For a process of
Subject; Thermodynamics
Using the book oh Thermodynamics. Cengel, Y.A., and Boles, M.A. (2008). Thermodynamics: An Engineering Approach. 6th ed. McGraw Hill.
For a process of combustion of Butane (formation enthalpy= -332,000 kJ/kmol) C4H10O with an excess of 25% of air (Reactives are in standard conditions of T=25 C, P= 1 atm)
Determine: A) The reason of air and fuel B) The reason of fuel and air C) The molar fractions of the product D) Temperature of the adiabatic flame E) If the products come out at a temperature of 600 K, determine de heat generated in the combustion chamber.
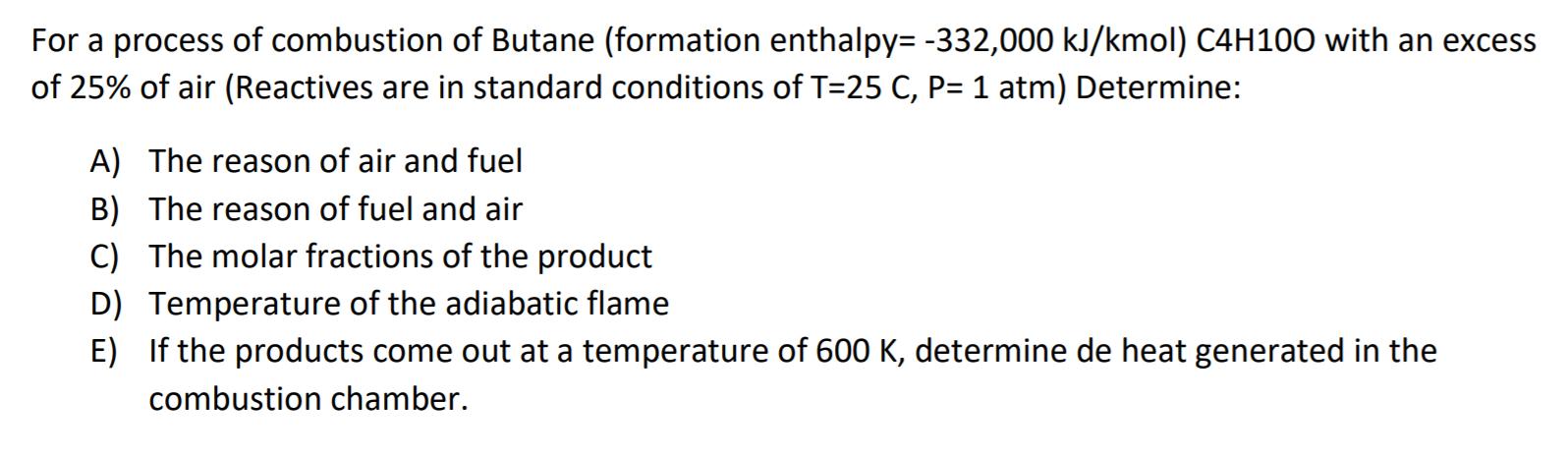
For a process of combustion of Butane (formation enthalpy= -332,000 kJ/kmol) C4H100 with an excess of 25% of air (Reactives are in standard conditions of T=25 C, P= 1 atm) Determine: A) The reason of air and fuel B) The reason of fuel and air C) The molar fractions of the product D) Temperature of the adiabatic flame E) If the products come out at a temperature of 600 K, determine de heat generated in the combustion chamber.
Step by Step Solution
3.49 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started