Question
Substance MgSO4(s) AH; (kJ/mol) -1278.2 AG; (kJ/mol) -1173.6 S (J/K-mol) 91.6 Mg+ (aq) -461.96 -456.0 -117.99 SO (aq) -907.5 -741.99 17.15 You write out



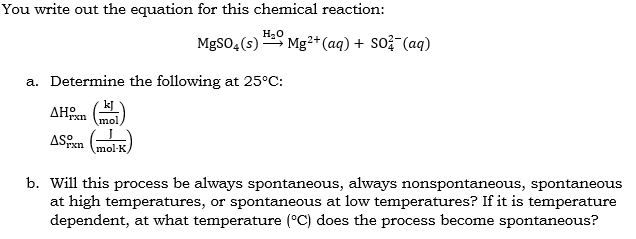
Substance MgSO4(s) AH; (kJ/mol) -1278.2 AG; (kJ/mol) -1173.6 S (J/K-mol) 91.6 Mg+ (aq) -461.96 -456.0 -117.99 SO (aq) -907.5 -741.99 17.15 You write out the equation for this chemical reaction: HO MgSO4(s) a. Determine the following at 25C: AH mol ASixn (mol-K) Mg2+ (aq) + SO2 (aq) b. Will this process be always spontaneous, always nonspontaneous, spontaneous at high temperatures, or spontaneous at low temperatures? If it is temperature dependent, at what temperature (C) does the process become spontaneous?
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Cost Accounting
Authors: William Lanen, Shannon Anderson, Michael Maher
3rd Edition
9780078025525, 9780077517359, 77517350, 978-0077398194
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



