Answered step by step
Verified Expert Solution
Question
1 Approved Answer
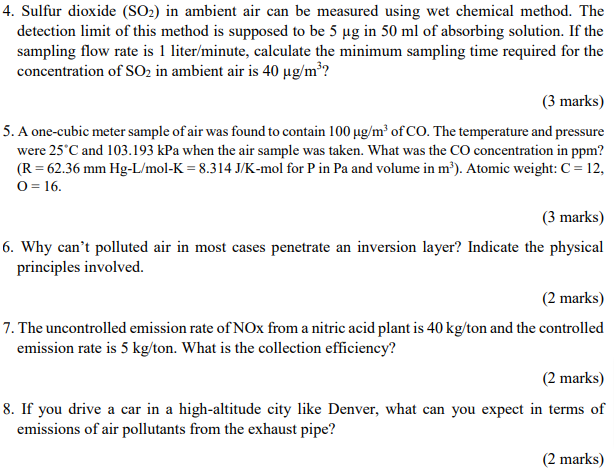
Sulfur dioxide ( S O 2 ) in ambient air can be measured using wet chemical method. The detection limit of this method is supposed
Sulfur dioxide in ambient air can be measured using wet chemical method. The
detection limit of this method is supposed to be in of absorbing solution. If the
sampling flow rate is literminute calculate the minimum sampling time required for the
concentration of in ambient air is
marks
A onecubic meter sample of air was found to contain of CO The temperature and pressure
were and kPa when the air sample was taken. What was the CO concentration in ppm
for in and volume in : Atomic weight:
marks
Why can't polluted air in most cases penetrate an inversion layer? Indicate the physical
principles involved.
marks
The uncontrolled emission rate of NOx from a nitric acid plant is ton and the controlled
emission rate is What is the collection efficiency?
marks
If you drive a car in a highaltitude city like Denver, what can you expect in terms of
emissions of air pollutants from the exhaust pipe?
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


