Question
Sulfuric acid has many applications, and is produced in greater amounts than any other chemical besides water. A high proportion of the manufactured sulfuric acid
Sulfuric acid has many applications, and is produced in greater amounts than any other chemical besides water. A high proportion of the manufactured sulfuric acid is used in the production of phosphate fertilizers and other uses include copper leaching, inorganic pigment production, petroleum refining, paper production, and industrial organic chemical production. It is forecasted that the global market size of sulfuric acid will be some 278 million tons in 2021. As a result it has become a challenge for global industrial players to develop a strategy to meet the target. Government of Ghana through Ministry of Trade and Industry and Ministry of Environment Sicience and Technology (MEST) has planned to set up 74 tons/day capacity sulfuric plant in Ghana. A key requirement is to design a sulfuric acid plant that will have a minimal emission of sulphur dioxide. Two main processes have been exploited to produce sulfuric acid worldwide. They are Lean Chamber and Contact processes. The lead chamber process, the older of the two processes, is used to produce much of the acid used to make fertilizers; it produces a relatively dilute acid (62%78% H 2SO 4). The contact process produces a purer, more concentrated acid but requires purer raw materials and the use of expensive catalysts. The later approach can be used to produce 93 to 98 or more percent H2SO4. However, the purity of the product will depend on starting materials (sulphur sources) and configuration of converter. In the contact process, the process plants are generally characterized according to the raw materials charged to them: (1) combustion of elemental sulfur, (2) combustion of spent sulfuric acid and hydrogen sulfide, and (3) combustion of metal sulfide ores and smelter gas burning. Sixty percent of the world's sulfuric acid is made from elemental sulfur. Virtually, all of this sulfur is the by-product of natural gas and petroleum refining. Figure A1 indicates a conventional process flow to produce sulfuric acid from elemental sulfur. The plant is installed to have single converter single absorber (SCSA) to produce 93 per cent (maximum for such a plant) sulfuric acid. The first step in the figure is combustion or oxidation of liquid sulphur which is classified to be combustible liquid. The sulfur in liquid or molten form is pumped from its storage vessel through heated lines and sprayed into a furnace using burners very similar to those usually used for burning fuel oil. The combustion products are always sulfur dioxide, oxygen, nitrogen, ash, CO2, water and carbon dusts. The hot gas is cooled and the heat recovery makes sulfuric acid plant based on this route more economical since the energy is used to generate high pressure steam for electricity production. The second step is purification of sulfur dioxide, a product which is characterised to support combustion of powdered aluminium and manganese and its contact with water results violent boiling where large heat is generated. The acceptable composition of feedstock to the converter, which serves as the heart hardware for production of sulfuric acid from elemental sulfur is 12 volume% SO2, 9 volume% O2, and 79 volume% N2 gas (420 C). To attain this composition, the combustion products are purified by using water scrubber to remove particulates as well a unwanted gases. It is further dried to remove water vapour using concentrated H2SO4 produced at absorption unit. Figure A1: SULFURIC ACID PLANT FROM COMBUSTION OF ELEMENTAL SULFUR Air blower or compressor is employed to pump dry air into the furnace. Sulfur - containg gas is propelled into a catalytic reactor packed with vanadium oxide as catalyst. The catalyst is loaded at 140 oC and reaction occurs at 450 oC: 2 + 22 23 + , this is the main reaction which converts sulfur dioxide into sulfur trioxide, and it is exothermic reversible reaction. As a result, the gas can exit at higher temperature usually 600oC or more.Sulfur trioxide the primary product of the converter is not combustible but is a fire risk when it comes into contact with organic materials such as wood, cotton, fiberboard etc. Sulphur trioxide has a strong affinity for water and may react with explosive violence with water to generate sulphuric acid. The product of the converter is cooled in an exchanger and finally entered absorber where conc sulfuric acid is sprayed over the gas to convert the sulfur trioxide gas to oleum acid. The olceum acid can further be diluted to meet the demand of clients at dilution tank. The sulfur dioxide gas is emitted to sodium carbonate scrubber for minimal emissions. Sulphuric acid as final product of the plant can result in violent reactions with water and strong bases generating heat. It is not compatible with organic materials, chlorates, carbides, fulminates, and powdered metals. In contact withmetal it releases flammable hydrogen gas that will explode if ignited in an enclosed area. The reaction equations for oleum, sulfuric acid and sulfur dioxide production are respectively given as: 3()++24() 227() 227 + 2 224 () + 2 2 Question 1: refer to figure A1 and any other data in the commentary to anticipate hazards (IDENTIFY TOXICANTS OR HAZADOUS SUBSTANCES AND OTHER HAZARDS) in the plant. Hint hazards include heat, thermal radiation, reactivity, noise and toxicant. Pick one toxicant or hazardous material, and write the following: 1. Health effects both acute and chronic 2. Threshold dose or values 3. LD/ LC fifty data 4. Engineering controls 5. Physical properties such as solubility, vapour density, boiling point, 6. Health and safety hazards e.g. carcinogenic, flammable etc. 7. Reactivity and stability data 8. First aid 9. PPE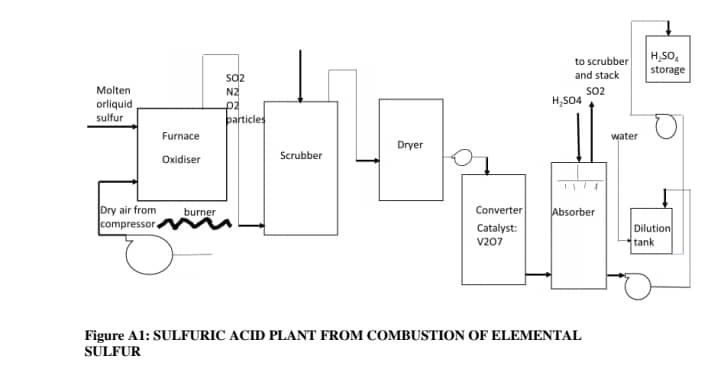
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


