Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Suppose 3 3 5 gallons of a mixture containing 4 w t % NaCl ( sodium chloride ) and 9 6 w t % water
Suppose gallons of a mixture containing NaCl sodium chloride and water solution density is mixed with an unknown volume of solution that is NaCl and water solution density Complete the questions to determine the volume of the second feed solution required to produce a mixture containing NaCl.
Label the flow chart of the mixing process. Do not leave any spaces empty.
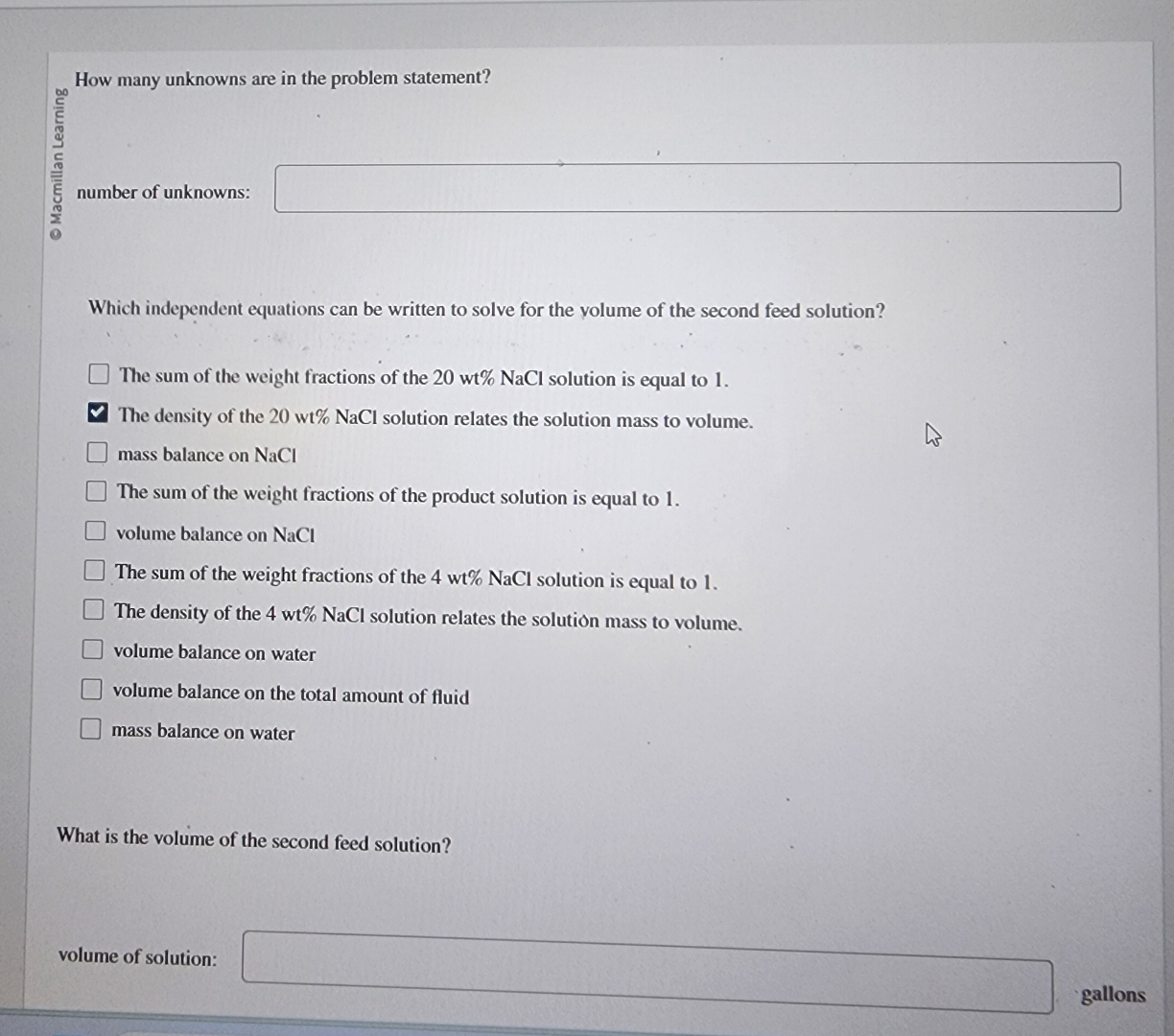
How many unknowns are in the problem statement?
number of unknowns:
Which independent equations can be written to solve for the yolume of the second feed solution?
The sum of the weight fractions of the NaCl solution is equal to
The density of the NaCl solution relates the solution mass to volume.
mass balance on NaCl
The sum of the weight fractions of the product solution is equal to
volume balance on NaCl
The sum of the weight fractions of the NaCl solution is equal to
The density of the NaCl solution relates the solution mass to volume.
volume balance on water
volume balance on the total amount of fluid
mass balance on water
What is the volume of the second feed solution?
volume of solution:
gallons
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


