Question
For the first order reaction: 2NOs(g) versus time data were collected at 45C and 65C Time(s) 4NO(g) + O(g), the following concentration 0 100
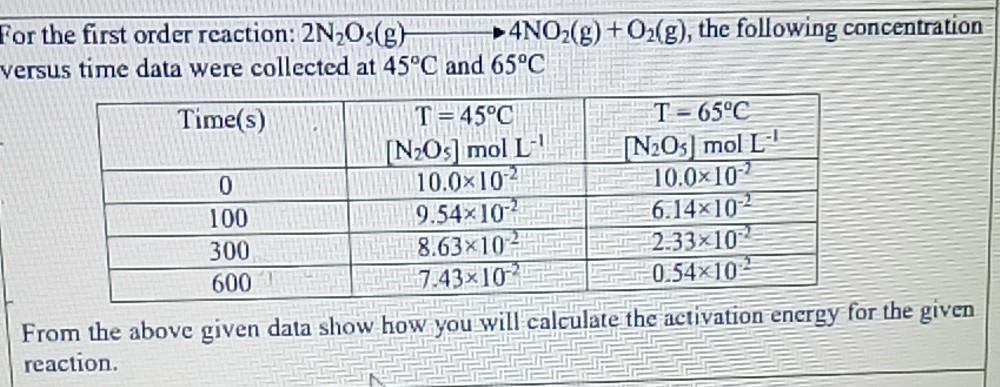
For the first order reaction: 2NOs(g) versus time data were collected at 45C and 65C Time(s) 4NO(g) + O(g), the following concentration 0 100 300 600 T C [NOs] mol L 10.010 9.5410 8.6310 7.43x102 T-65C [NO5 mol L 10.010-2 6.14102 2.33x102 0.54x102 From the above given data show how you will calculate the activation energy for the given reaction.
Step by Step Solution
3.57 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Step1 For firstorder reactions the differential rate equation is rate A rate k A or dAdt kA The inte...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Project Management A Managerial Approach
Authors: Jack R. Meredith, Samuel J. Mantel,
7th Edition
470226218, 978-0470226216
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



