Question
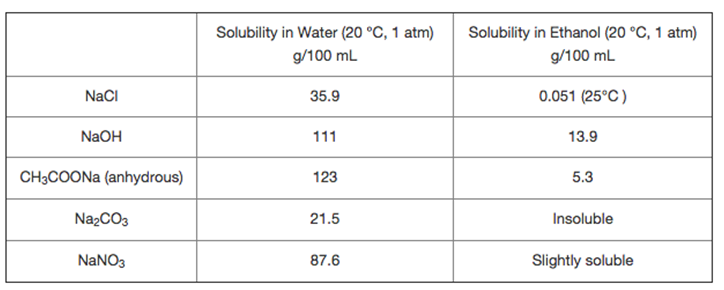
Suppose you need to differentiate the solids in each pair using their solubility difference in water and ethanol (as shown in the table). So you
Suppose you need to differentiate the solids in each pair using their solubility difference in water and ethanol (as shown in the table). So you dissolve 0.40 g of each solid into 10 mL each solvent (Each solution composes one solvent and one solute). The solids in which pair CANNOT be differentiated using their solubility difference in water and ethanol?
- Na 2 CO 3 and CH 3 COONa (anhydrous)
- NaOH and NaOH 3
- NaCI and NaOH
- NaOH and CH 3 COONa (anhydrous)
- NaOH 3 and CH 3 COONa (anhydrous)
NaCl NaOH CH3COONa (anhydrous) NaCO3 NaNO3 Solubility in Water (20 C, 1 atm) g/100 mL 35.9 111 123 21.5 87.6 Solubility in Ethanol (20 C, 1 atm) g/100 mL 0.051 (25C) 13.9 5.3 Insoluble Slightly soluble
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
NaOH and CH3COONa anhydrous For both NaOH and CH3COONa anhydr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Money Banking and Financial Markets
Authors: Stephen Cecchetti, Kermit Schoenholtz
4th edition
007802174X, 978-0078021749
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



