Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Symbol Name 17C 13C 22Ne 345 Ca Kr 94Zr carbon-12 carbon-13 neon-22 neon-21 sulfur-34 chlorine-37 krypton-82 zirconium-94 Zn 672n 56Fe 55Mn 42Ca2+ 1271 lodine-127
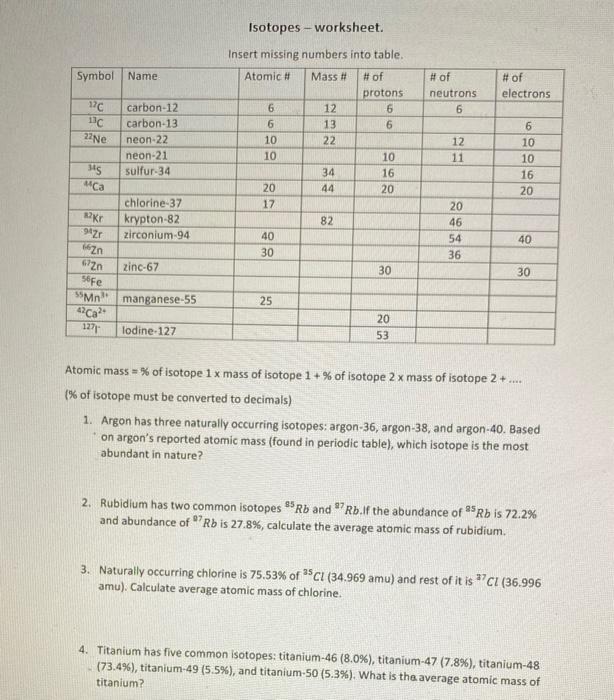
Symbol Name 17C 13C 22Ne 345 Ca Kr 94Zr carbon-12 carbon-13 neon-22 neon-21 sulfur-34 chlorine-37 krypton-82 zirconium-94 Zn 672n 56Fe 55Mn 42Ca2+ 1271 lodine-127 zinc-67 manganese-55 Isotopes - worksheet. Insert missing numbers into table. Atomic # Mass # # of protons 6 6 6 10 10 20 17 40 30 25 232 12 13 34 44 82 6 10 16 20 30 20 53 # of neutrons 6 12 11 20 46 54 36 # of electrons 6 10 10 16 20 40 30 Atomic mass=% of isotope 1 x mass of isotope 1+% of isotope 2 x mass of isotope 2+.... (% of isotope must be converted to decimals) 1. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon's reported atomic mass (found in periodic table), which isotope is the most abundant in nature? 2. Rubidium has two common isotopes 85Rb and 7Rb.If the abundance of 25 Rb is 72.2% and abundance of 7 Rb is 27.8%, calculate the average atomic mass of rubidium. 3. Naturally occurring chlorine is 75.53% of 35Cl (34.969 amu) and rest of it is 7 Cl (36.996 amu). Calculate average atomic mass of chlorine. 4. Titanium has five common isotopes: titanium-46 (8.0%), titanium-47 (7.8%), titanium-48 (73.4%), titanium-49 (5.5%), and titanium-50 (5.3%). What is the average atomic mass of titanium?
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Answer Symbol Name 17C 13C 22Ne 21 Ne 345 MCa 37 CI 82Kr 94Zr 66Zn 672n 56FC 55Mn 42Ca2 1271 carbon1...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


