Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Table 9-1: Dissolved Oxygen (DO) for BOD samples Initial DO concentration DO concentration after 5 days Sample (mg/L) (mg/L) Trial 1 Trial 2 Trial
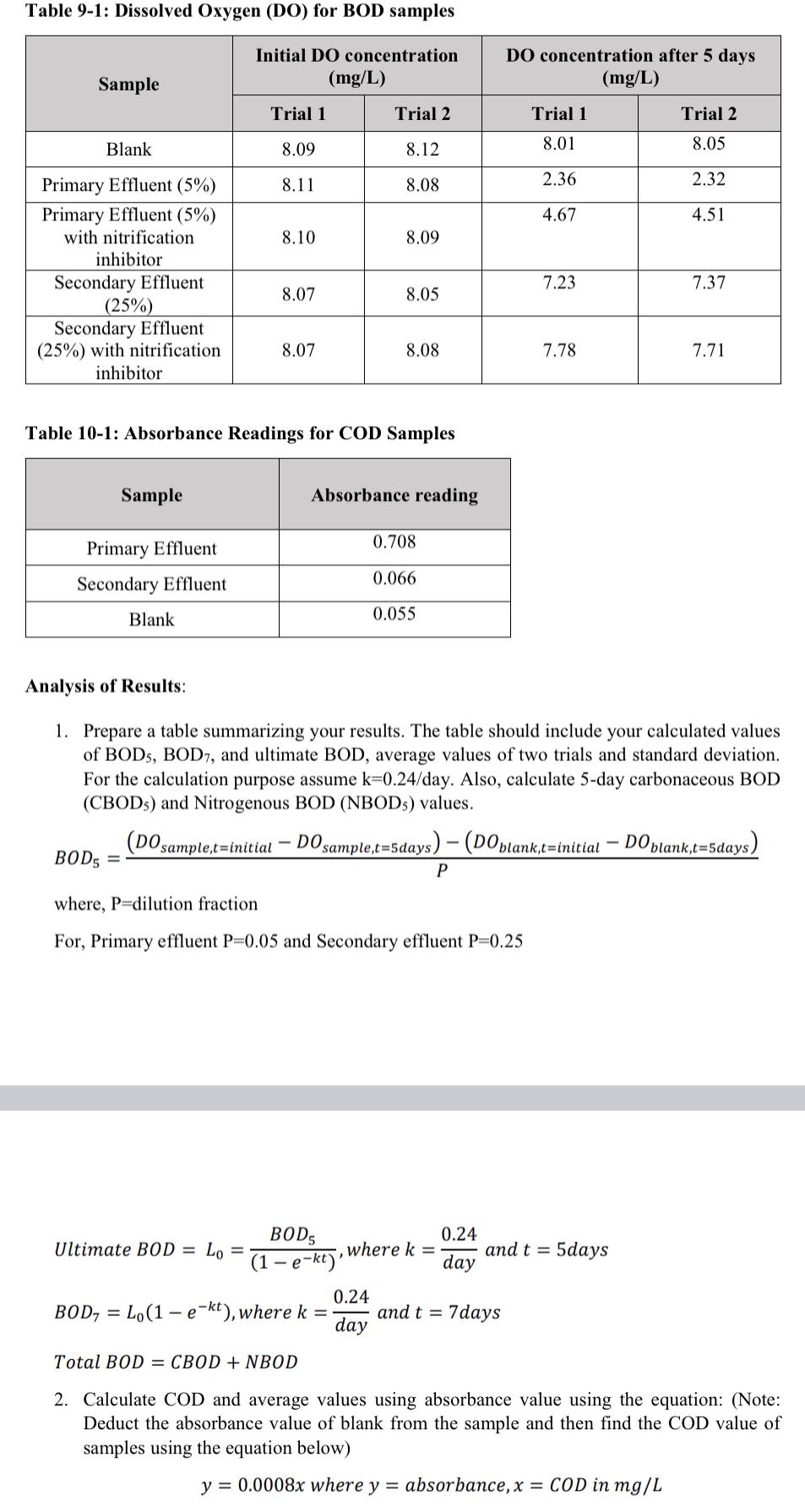
Table 9-1: Dissolved Oxygen (DO) for BOD samples Initial DO concentration DO concentration after 5 days Sample (mg/L) (mg/L) Trial 1 Trial 2 Trial 1 Trial 2 Blank 8.09 8.12 8.01 8.05 Primary Effluent (5%) 8.11 2.36 2.32 8.08 Primary Effluent (5%) 4.67 4.51 with nitrification 8.10 8.09 inhibitor Secondary Effluent 7.23 7.37 8.07 8.05 (25%) Secondary Effluent (25%) with nitrification. 8.07 8.08 7.78 7.71 inhibitor Table 10-1: Absorbance Readings for COD Samples Sample Absorbance reading Primary Effluent 0.708 Secondary Effluent 0.066 Blank 0.055 Analysis of Results: 1. Prepare a table summarizing your results. The table should include your calculated values of BOD5, BOD7, and ultimate BOD, average values of two trials and standard deviation. For the calculation purpose assume k=0.24/day. Also, calculate 5-day carbonaceous BOD (CBODs) and Nitrogenous BOD (NBOD5) values. (DO sample,t=initial BOD5 = DO sample,t=5days) - (DO blank,t-initial DO blank,t=5days) P where, P=dilution fraction For, Primary effluent P=0.05 and Secondary effluent P=0.25 Ultimate BOD = L = BOD5 (1-e-kt)' 0.24 where k = and t = 5days day 0.24 = BOD, Lo(1-e-kt), where k = and t = 7days day Total BOD CBOD+NBOD 2. Calculate COD and average values using absorbance value using the equation: (Note: Deduct the absorbance value of blank from the sample and then find the COD value of samples using the equation below) y = 0.0008x where y = absorbance, x = COD in mg/L
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


