Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The figure provides the T-s diagram of a Carnot heat pump cycle for which the substance is ammonia. Determine the net work input required,
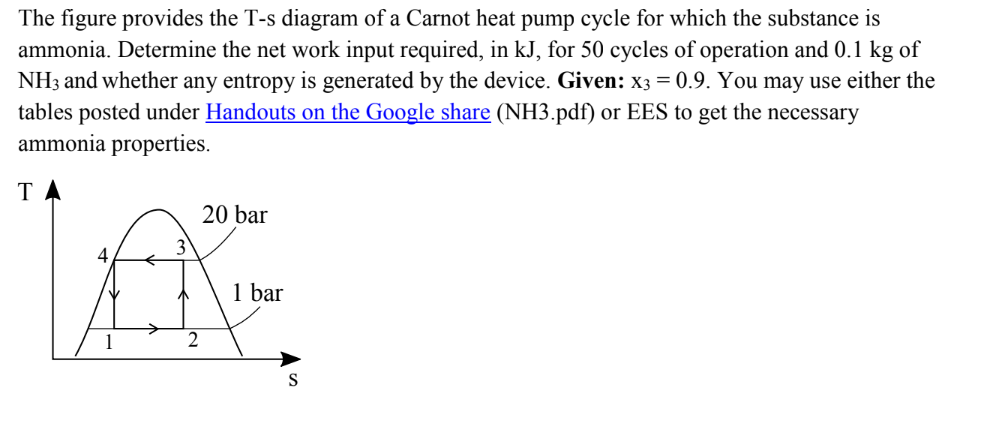
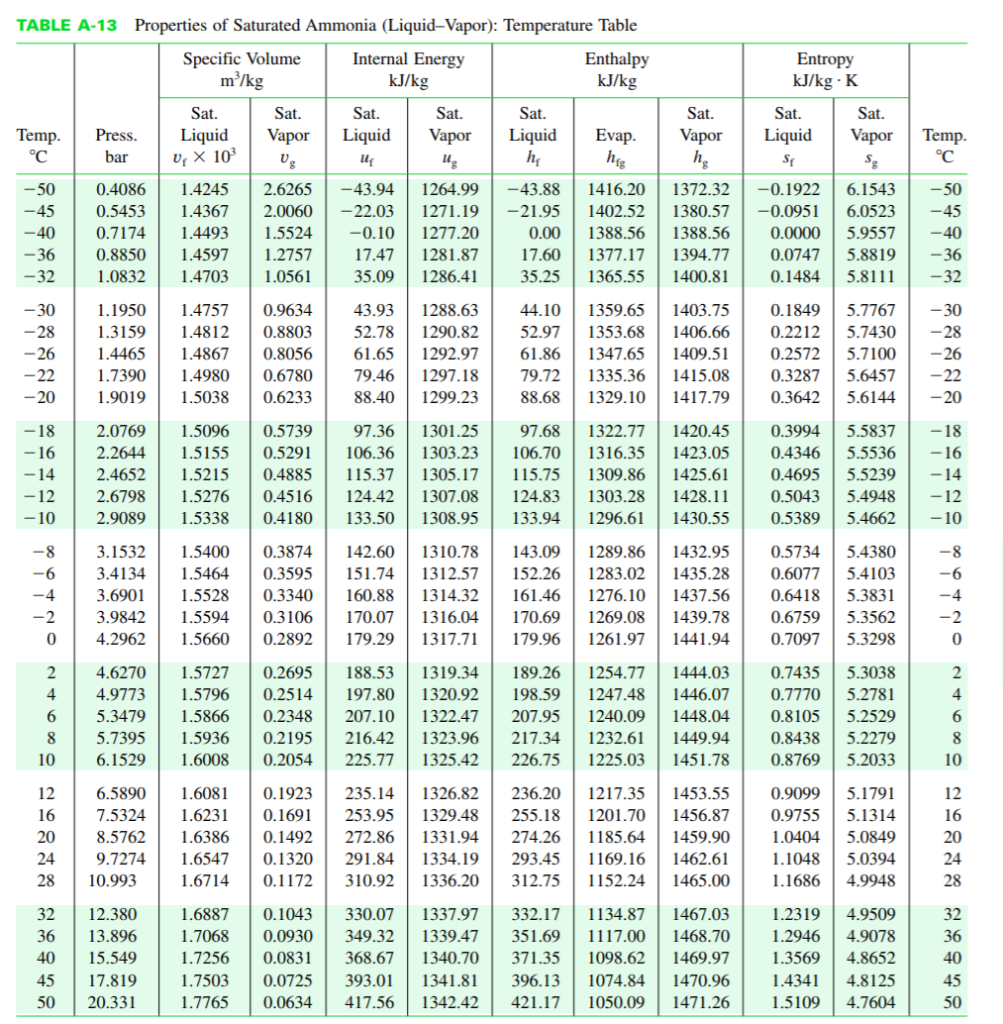
The figure provides the T-s diagram of a Carnot heat pump cycle for which the substance is ammonia. Determine the net work input required, in kJ, for 50 cycles of operation and 0.1 kg of NH3 and whether any entropy is generated by the device. Given: x3 = 0.9. You may use either the tables posted under Handouts on the Google share (NH3.pdf) or EES to get the necessary ammonia properties. 2 20 bar 1 bar S TABLE A-13 Properties of Saturated Ammonia (Liquid-Vapor): Temperature Table Specific Volume Internal Energy m/kg kJ/kg Temp. -50 -45 -40 -36 -32 -30 -28 -26 -22 -20 -8 -6 -4 -2 0 +680 DEGRE 5 -18 2.0769 -16 2.2644 1.5155 -14 -12 2.4652 1.5215 2.6798 1.5276 2.9089 1.5338 -10 10 Sat. Press. Liquid bar U X 10 12 16 2 4.6270 1.5727 0.2695 4 4.9773 1.5796 0.2514 0.2348 0.2195 0.2054 5.3479 1.5866 5.7395 1.5936 6.1529 1.6008 20 Sat. Vapor Ug 1.1950 1.4757 0.9634 1.3159 1.4812 0.8803 1.4465 1.4867 0.8056 1.7390 1.4980 0.6780 1.9019 1.5038 0.6233 Sat. Liquid U Sat. Vapor Ug 32 12.380 36 13.896 40 15.549 45 17.819 50 20.331 1.5096 0.5739 97.36 1301.25 0.5291 106.36 1303.23 0.4885 115.37 1305.17 0.4516 124.42 1307.08 0.4180 133.50 1308.95 0.4086 1.4245 2.6265 -43.94 1264.99 -43.88 1416.20 0.5453 1.4367 2.0060 -22.03 1271.19 -21.95 1402.52 0.7174 1.4493 1.5524 -0.10 1277.20 0.00 1388.56 1388.56 0.8850 1.4597 1.2757 17.47 1281.87 17.60 1377.17 1394.77 1.0832 1.4703 1.0561 35.09 1286.41 35.25 1365.55 1400.81 44.10 1359.65 1403.75 52.97 1353.68 1406.66 61.86 1347.65 1409.51 79.72 1335.36 1415.08 88.68 1329.10 1417.79 43.93 1288.63 52.78 1290.82 61.65 1292.97 79.46 1297.18 88.40 1299.23 Sat. Liquid h Enthalpy kJ/kg Evap. hig Sat. Vapor hg 1.6887 0.1043 330.07 1337.97 1.7068 0.0930 349.32 1339.47 1.7256 0.0831 368.67 1340.70 1.7503 0.0725 393.01 1341.81 1.7765 0.0634 417.56 1342.42 143.09 1289.86 1432.95 1283.02 1435.28 3.1532 1.5400 0.3874 142.60 1310.78 3.4134 1.5464 0.3595 151.74 1312.57 152.26 3.6901 1.5528 0.3340 160.88 1314.32 161.46 1276.10 1437.56 3.9842 1.5594 0.3106 170.07 1316.04 170.69 1269.08 1439.78 4.2962 1.5660 0.2892 179.29 1317.71 179.96 1261.97 1441.94 6.5890 1.6081 236.20 1217.35 1453.55 0.1923 235.14 1326.82 0.1691 253.95 1329.48 255.18 1201.70 1456.87 7.5324 1.6231 8.5762 1.6386 0.1492 272.86 1331.94 24 1.6547 0.1320 9.7274 28 10.993 291.84 1334.19 310.92 1336.20 274.26 1185.64 1459.90 293.45 1169.16 1462.61 312.75 1152.24 1465.00 1.6714 0.1172 97.68 1322.77 1420.45 106.70 1316.35 1423.05 115.75 1309.86 1425.61 124.83 1303.28 1428.11 133.94 1296.61 1430.55 1372.32 -0.1922 6.1543 1380.57 -0.0951 6.0523 0.0000 5.9557 0.0747 5.8819 0.1484 5.8111 188.53 1319.34 189.26 1254.77 1444.03 197.80 1320.92 198.59 1247.48 1446.07 207.10 1322.47 207.95 1240.09 1448.04 216.42 1323.96 217.34 1232.61 1449.94 225.77 1325.42 226.75 1225.03 1451.78 332.17 1134.87 1467.03 351.69 1117.00 1468.70 371.35 1098.62 1469.97 Entropy kJ/kg - K 396.13 1074.84 1470.96 421.17 1050.09 1471.26 Sat. Liquid SE Sat. Vapor Sg 0.1849 5.7767 0.2212 5.7430 0.2572 5.7100 0.3287 5.6457 0.3642 5.6144 0.3994 5.5837 0.4346 5.5536 0.4695 5.5239 0.5043 0.5389 0.5734 5.4380 0.6077 5.4103 0.6418 5.3831 0.6759 5.3562 0.7097 5.3298 0.7435 5.3038 0.7770 5.2781 0.8105 5.2529 0.8438 5.2279 0.8769 5.2033 0.9099 5.1791 0.9755 5.1314 1.0404 5.0849 1.1048 5.0394 1.1686 4.9948 1.2319 1.2946 1.3569 4.8652 1.4341 4.8125 1.5109 4.7604 Temp. C 4.9509 4.9078 -50 -45 -14 -12 5.4948 5.4662 -10 -40 -36 -32 -30 -28 -26 -22 -20 -18 -16 -8 24823 55 5AN ONA -6 -4 -2 28 45 50
Step by Step Solution
★★★★★
3.33 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


