Answered step by step
Verified Expert Solution
Question
1 Approved Answer
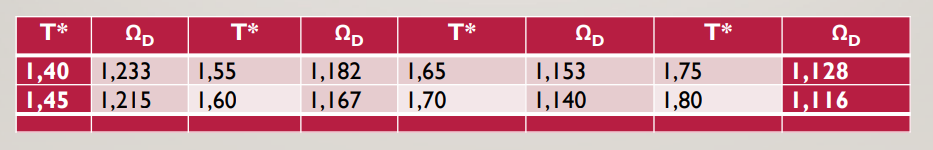
table [ [ T ^ ( * * ) , Omega _ ( D ) , T ^ ( * * ) ,
tableTOmega DTOmega DTOmega DTOmega DII,I,I,II,I,II,I,III,II,I,II,I,II,I,IICalculate the diffusion of atm methanol A vapors at deg C to air B using the ChapmanEnskog relation. The average molecular diameter of air is nm nmxcmepsi Bk K mole weight is ggmol normal boiling point of methanol is deg C Mole weight of methanol is g gmol and The atomic volume at normal boiling point will be taken as cm gmol for C cm gmol for H and cm gmol for O
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


