Answered step by step
Verified Expert Solution
Question
1 Approved Answer
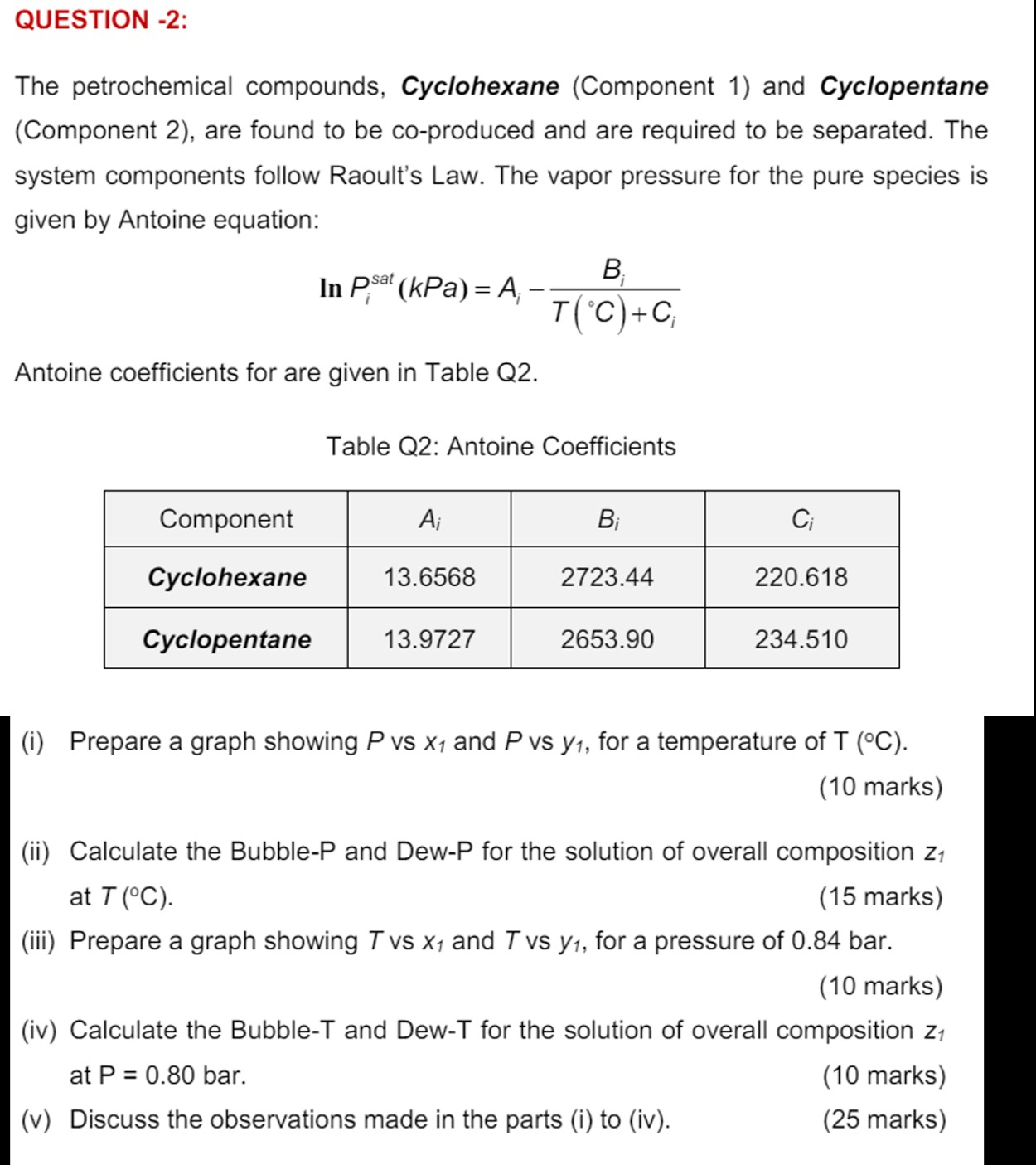
take z 1 = 0 . 6 8 and T ( C ) = 1 2 9 , All graphs should be plotted using Microsoft
take z and TC All graphs should be plotted using Microsoft Excel please show the solution typed and show all calculation steps. thank u so much for your help, I will upvot :
QUESTION :the petrochemical compounds, Cyclohexane Component and Cyclopentane
Component are found to be coproduced and are required to be separated. The
system components follow Raoult's Law. The vapor pressure for the pure species is
given by Antoine equation:
Antoine coefficients for are given in Table Q
Table Q: Antoine Coefficients
i Prepare a graph showing vs and vs for a temperature of
marks
ii Calculate the BubbleP and DewP for the solution of overall composition
at
marks
iii Prepare a graph showing vs and vs for a pressure of bar.
marks
iv Calculate the BubbleT and DewT for the solution of overall composition
at bar.
marks
v Discuss the observations made in the parts i to iv
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


