Question
1) Consider the structure shown below... (19 pts total) N - H bond N - C bond C-C3 bond 1 H (Shouldn't be bent.
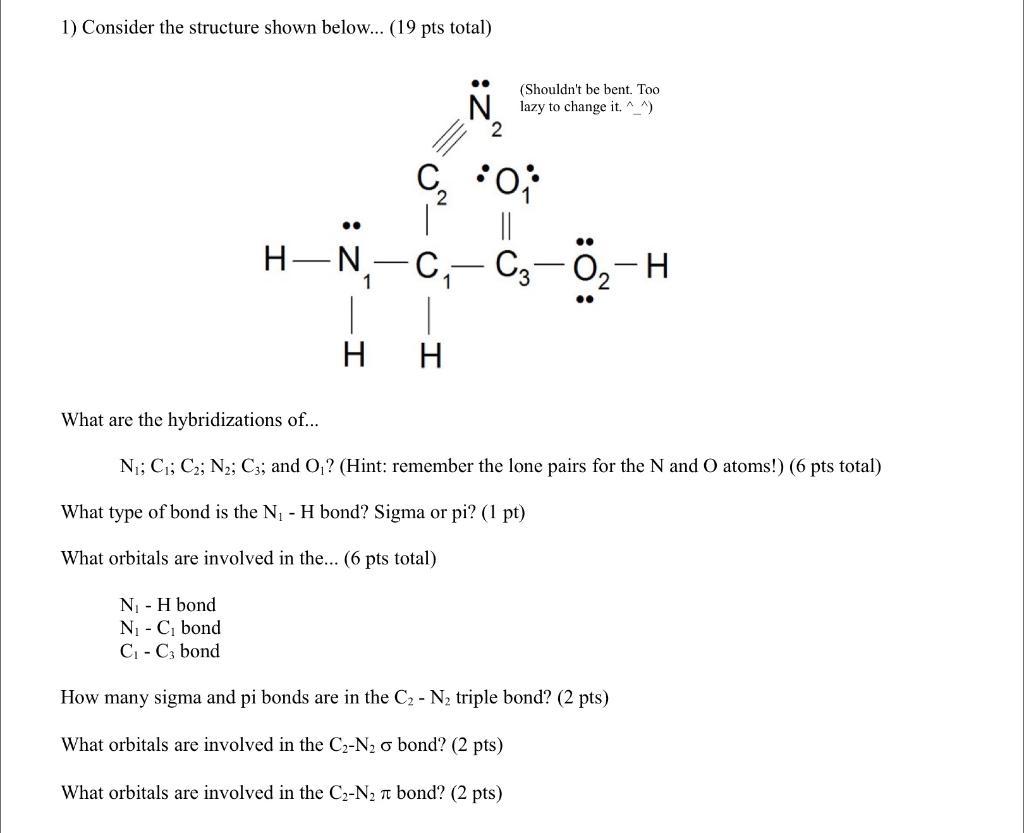
1) Consider the structure shown below... (19 pts total) N - H bond N - C bond C-C3 bond 1 H (Shouldn't be bent. Too Nlazy to change it. ^_^) 2 *O* C H-N-C-C3-0-H H What are the hybridizations of... N; C; C2; N2; C3; and O? (Hint: remember the lone pairs for the N and O atoms!) (6 pts total) What type of bond is the N - H bond? Sigma or pi? (1 pt) What orbitals are involved in the... (6 pts total) How many sigma and pi bonds are in the C - N triple bond? (2 pts) What orbitals are involved in the C-N o bond? (2 pts) What orbitals are involved in the C-N T bond? (2 pts)
Step by Step Solution
3.47 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
Ans Hybridization into new It involves ID G iii C iv N V C8 HN ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting
Authors: Robert Libby, Patricia Libby, Daniel Short
8th edition
78025559, 978-0078025556
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



