Question
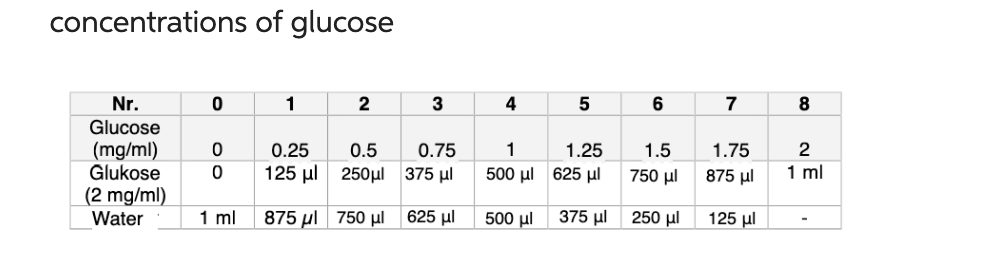
Task 1) - Glucose calibration curve: a) plot the measured absorbances (y-axis) against the glucose concentrations (indicated in mg/ml, mg glucose/reaction preparation and glucose concentration
Task 1) - Glucose calibration curve:
a) plot the measured absorbances (y-axis) against the glucose concentrations (indicated in mg/ml, mg glucose/reaction preparation and glucose concentration in mM) (x-axis).
b) plot the measured absorbances (y-axis) against time (x-axis), use one and the same diagram for all four curves!
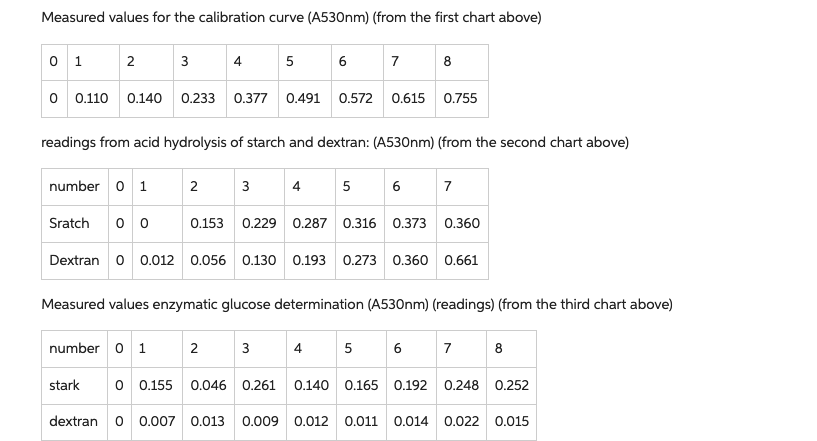
c) From the plateau of the curves, determine the values with the highest absorbance (end point) and use the glucose calibration curve to determine the amount of glucose formed during hydrolysis!
d) Use the value of batch 8 of the acid hydrolysis as the value of the maximum conversion (100 % = complete cleavage of glycogen into glucose) and calculate the percentage value of the maximum conversion for the enzymatic hydrolysis! maximum conversion!
e) What is the maximum amount of glucose that could have been released? Compare this value with the amount of glucose released during acidic hydrolysis!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


