Answered step by step
Verified Expert Solution
Question
1 Approved Answer
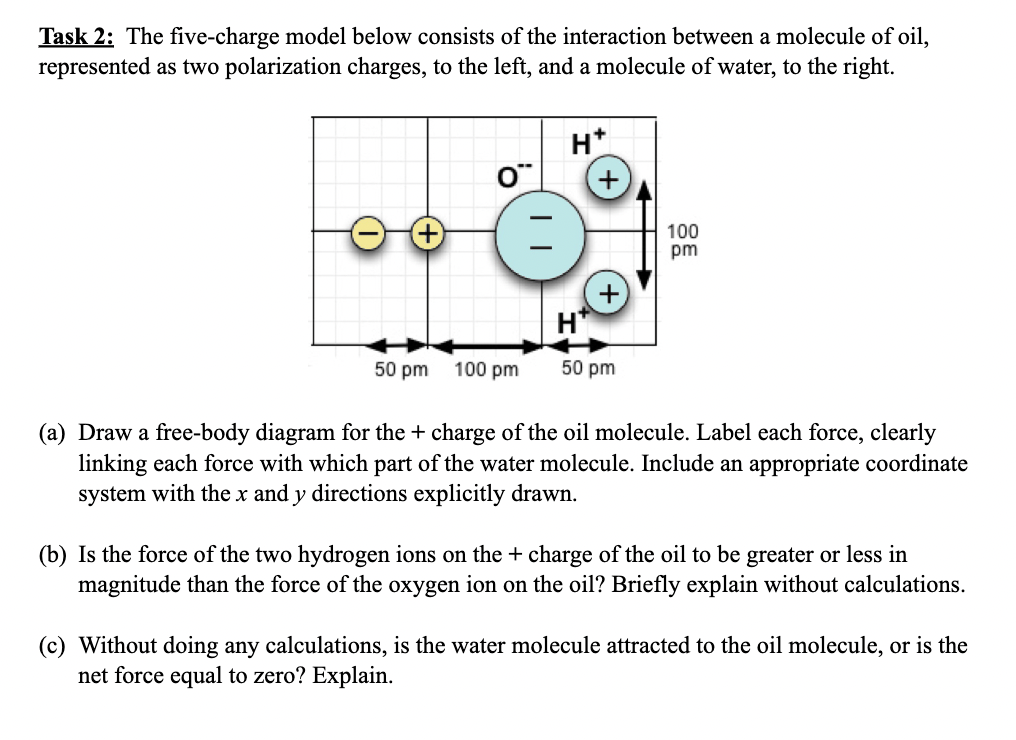
Task 2 : The five - charge model below consists of the interaction between a molecule of oil, represented as two polarization charges, to the
Task : The fivecharge model below consists of the interaction between a molecule of oil,
represented as two polarization charges, to the left, and a molecule of water, to the right.
a Draw a freebody diagram for the charge of the oil molecule. Label each force, clearly
linking each force with which part of the water molecule. Include an appropriate coordinate
system with the and directions explicitly drawn.
b Is the force of the two hydrogen ions on the charge of the oil to be greater or less in
magnitude than the force of the oxygen ion on the oil? Briefly explain without calculations.
c Without doing any calculations, is the water molecule attracted to the oil molecule, or is the
net force equal to zero? Explain.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


