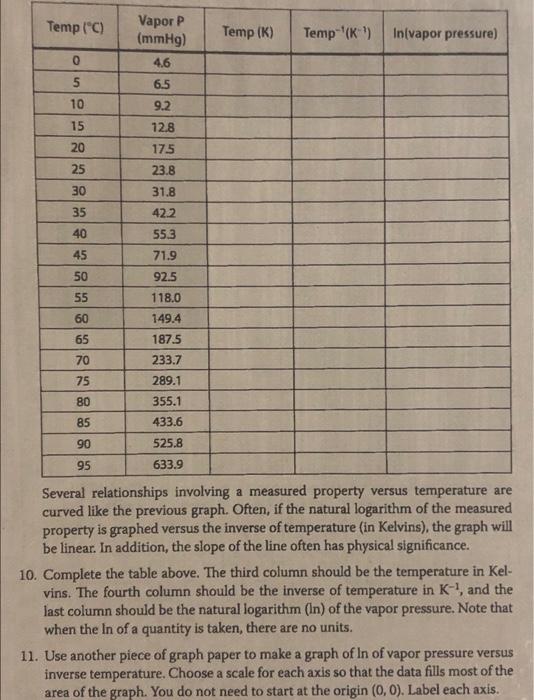
Temp (C) Temp (K) Temp-'(K) In(vapor pressure) 0 Vapor P (mmHg) 4.6 6.5 9.2 12.8 5 10 15 20 175 25 23.8 30 31.8 35 422 55.3 40 45 71.9 50 92.5 55 118.0 149.4 60 65 187.5 233.7 70 75 289.1 355.1 80 85 433.6 525.8 90 95 633.9 Several relationships involving a measured property versus temperature are curved like the previous graph. Often, if the natural logarithm of the measured property is graphed versus the inverse of temperature (in Kelvins), the graph will be linear. In addition, the slope of the line often has physical significance. 10. Complete the table above. The third column should be the temperature in Kel- vins. The fourth column should be the inverse of temperature in K, and the last column should be the natural logarithm (In) of the vapor pressure. Note that when the In of a quantity is taken, there are no units. 11. Use another piece of graph paper to make a graph of In of vapor pressure versus inverse temperature. Choose a scale for each axis so that the data fills most of the area of the graph. You do not need to start at the origin (0, 0). Label each axis. a 12 Plot each of the data points, and circle each point. The data should be linear Use a see-through straight edge to draw a straight line that best fits the data. Put a title on the graph. 13. Calculate the slope of the line. Choose two points on the line that are on the cor- ners of the grid, and far apart. Do not use data points to calculate the slope Indicate the two points used to calculate the slope on the graph. Show the calcu- lation of the slope below. Note that the In of P has no units, but 1/T has the units of K-1. As a result, the slope will have units. Slope 14. The slope of the line has physical significance. The slope is used to calculate the enthalpy of vaporization, AH The enthalpy of vaporization is the energy need- ed to vaporize a mole of a substance at its normal boiling point Slope = -AH/R Where R = 8.314 J/mol-K Show the calculation of the enthalpy of vaporization below. Pay attention to the sign and units. Result: AH. 15. Look up the value of the enthalpy of vaporization of water in your textbook. Cal- culate the % error using the value from your graph and the accepted value. Show your calculation below. | AH., from graph--actual AH %error = X 100% actual AH.. Result: % error