Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Test Content 30 Points Question 1 100 moles of liquid methanoi at 25C is burned in a combustion chamber with 100% excess air. Assuming complete
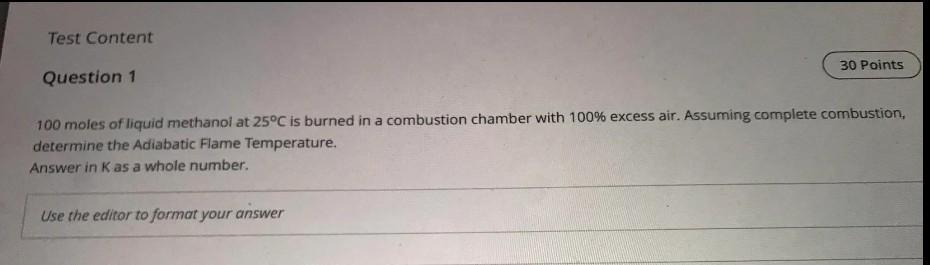
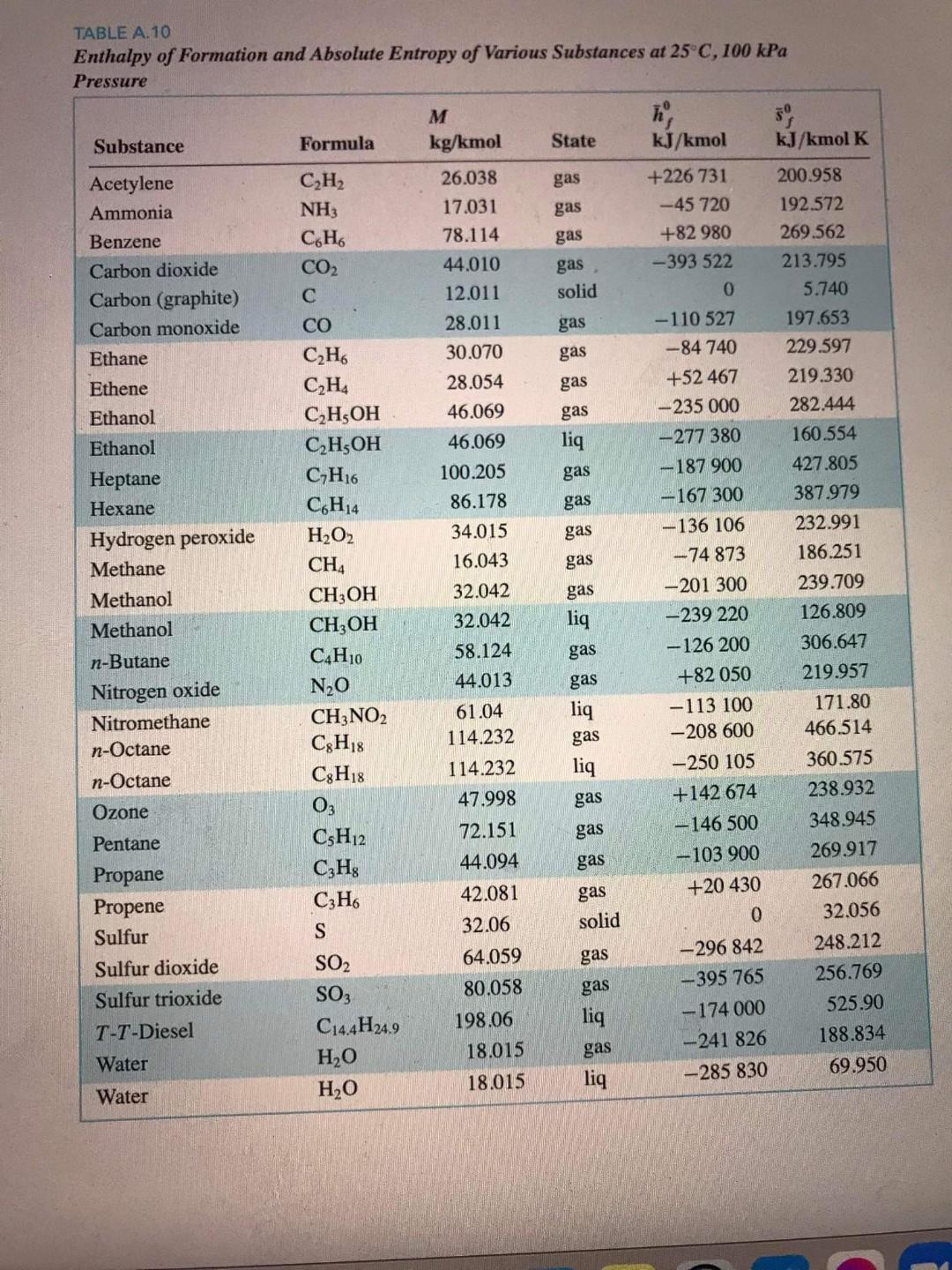
Test Content 30 Points Question 1 100 moles of liquid methanoi at 25C is burned in a combustion chamber with 100% excess air. Assuming complete combustion, determine the Adiabatic Flame Temperature. Answer in K as a whole number. Use the editor to format your answer TABLE A.10 Enthalpy of Formation and Absolute Entropy of Various Substances at 25 C, 100 kPa Pressure Substance Formula State kJ/kmol kJ/kmol K gas gas gas gas solid gas gas gas gas liq gas gas Acetylene Ammonia Benzene Carbon dioxide Carbon (graphite) Carbon monoxide Ethane Ethene Ethanol Ethanol Heptane Hexane Hydrogen peroxide Methane Methanol Methanol n-Butane Nitrogen oxide Nitromethane n-Octane n-Octane Ozone Pentane Propane Propene Sulfur Sulfur dioxide Sulfur trioxide T-T-Diesel gas gas 200.958 192.572 269.562 213.795 5.740 197.653 229.597 219.330 282.444 160.554 427.805 387.979 232.991 186.251 239.709 126.809 M kg/kmol 26.038 17.031 78.114 44.010 12.011 28.011 30.070 28.054 46.069 46.069 100.205 86.178 34.015 16.043 32.042 32.042 58.124 44.013 61.04 114.232 114.232 47.998 72.151 44.094 42.081 32.06 64.059 80.058 198.06 18.015 C2H2 NH3 C.H. CO2 C2H C2H4 C2H5OH C2H5OH CH16 C6H14 H2O2 CH CH3OH CH3OH C4H10 N20 CH3NO2 C3H18 C3H18 03 C3H12 CzHg C3H6 S SO2 SO3 C14.4H24.9 HO H2O +226 731 -45 720 +82 980 -393 522 0 -110 527 -84 740 +52 467 -235 000 -277 380 -187 900 -167 300 -136 106 -74 873 -201 300 -239 220 -126 200 +82 050 -113 100 -208 600 -250 105 +142 674 -146 500 -103 900 +20 430 0 306.647 219.957 gas liq gas gas liq gas liq gas gas gas gas solid 171.80 466.514 360.575 238.932 348.945 269.917 267.066 32.056 248.212 256.769 525.90 188.834 69.950 gas gas liq gas liq -296 842 -395 765 -174 000 -241 826 -285 830 Water Water 18.015
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


