Answered step by step
Verified Expert Solution
Question
1 Approved Answer
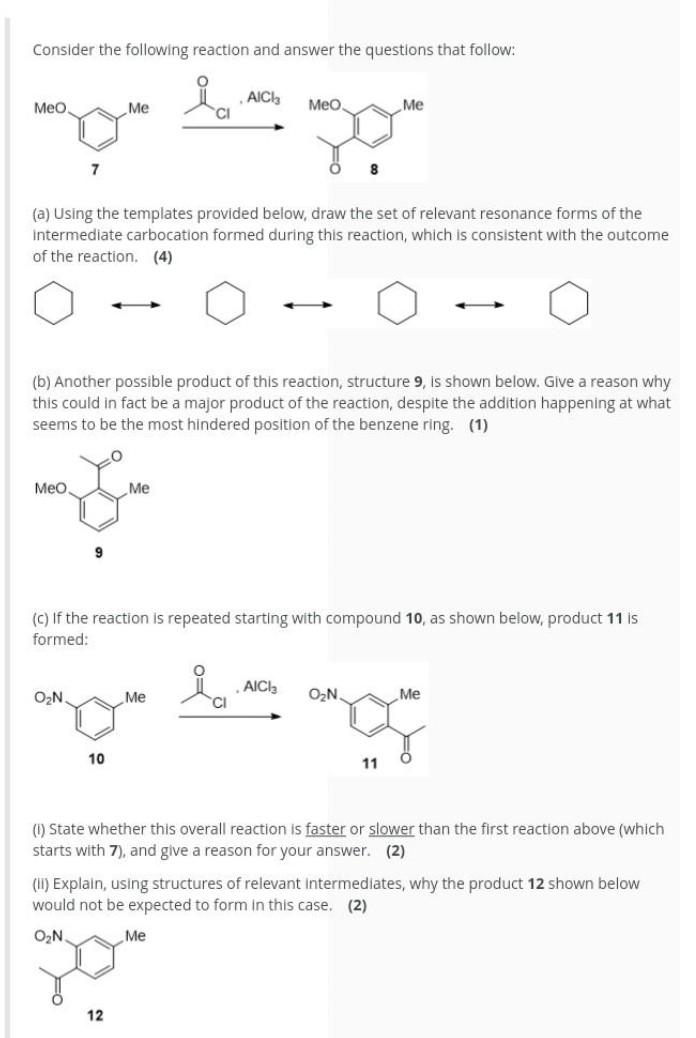
Thank you Consider the following reaction and answer the questions that follow: ia Meo Me AICI: CI Meo Me 7 8 (a) Using the templates
Thank you
Consider the following reaction and answer the questions that follow: ia Meo Me AICI: CI Meo Me 7 8 (a) Using the templates provided below, draw the set of relevant resonance forms of the intermediate carbocation formed during this reaction, which is consistent with the outcome of the reaction. (4) (b) Another possible product of this reaction, structure 9, is shown below. Give a reason why this could in fact be a major product of the reaction, despite the addition happening at what seems to be the most hindered position of the benzene ring. (1) Meo Me (c) If the reaction is repeated starting with compound 10, as shown below, product 11 is formed: AICI ON Me ON Me 10 11 (l) State whether this overall reaction is faster or slower than the first reaction above (which starts with 7), and give a reason for your answer. (2) (II) Explain, using structures of relevant intermediates, why the product 12 shown below would not be expected to form in this case. (2) O2N Me 12Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


