Answered step by step
Verified Expert Solution
Question
1 Approved Answer
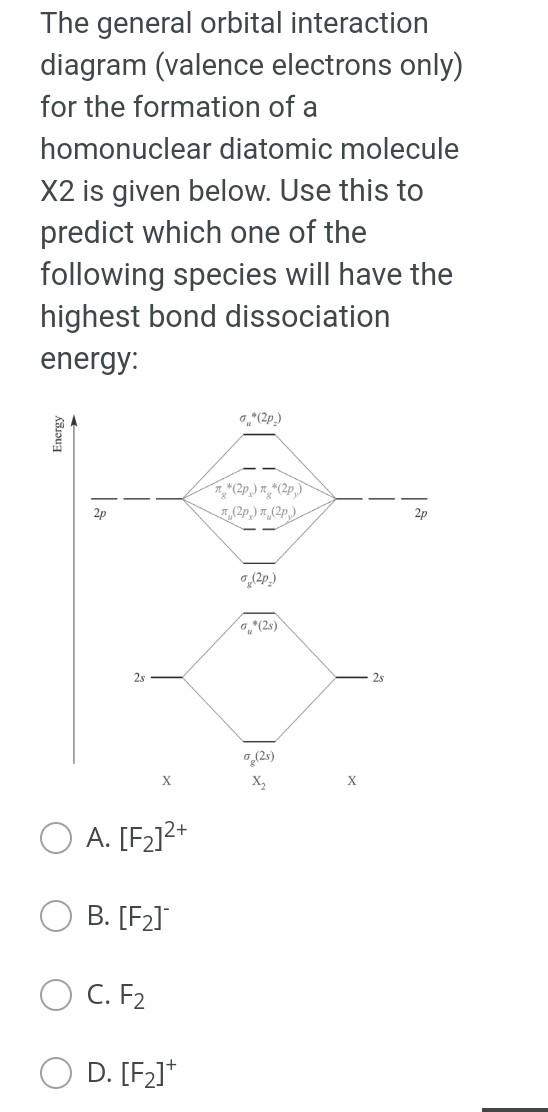
Thank you The general orbital interaction diagram (valence electrons only) for the formation of a homonuclear diatomic molecule X2 is given below. Use this to

Thank you
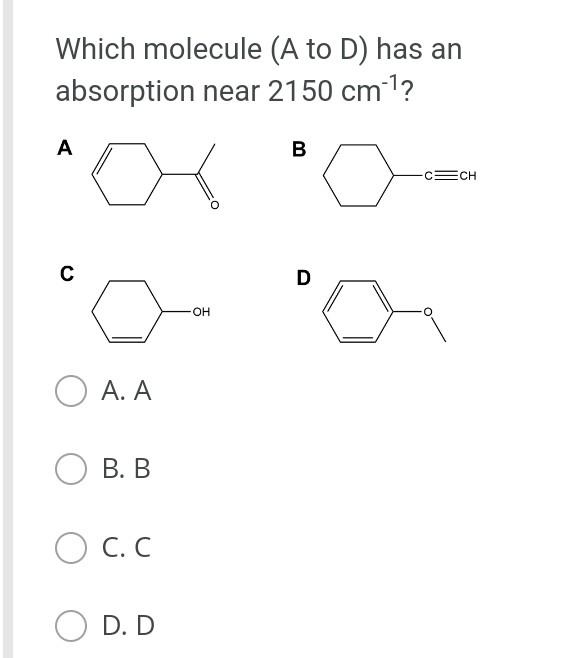
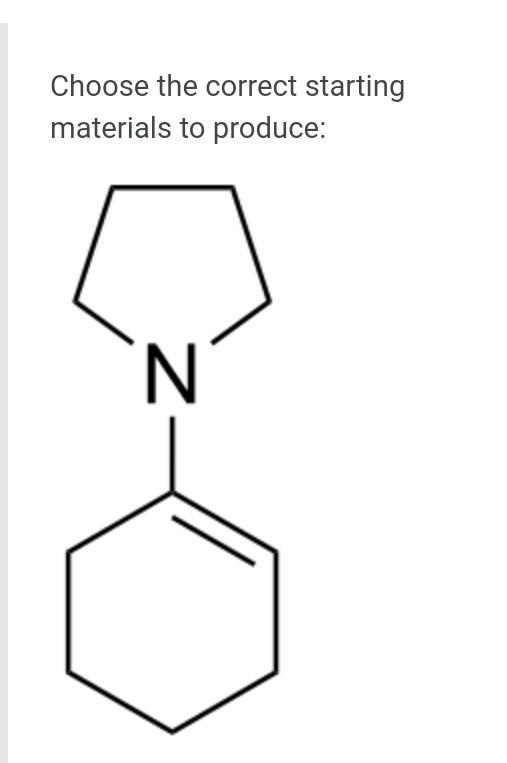
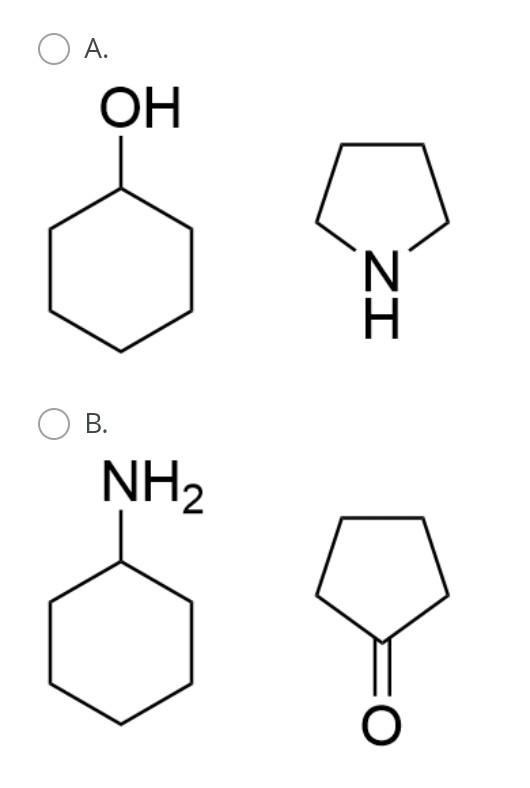
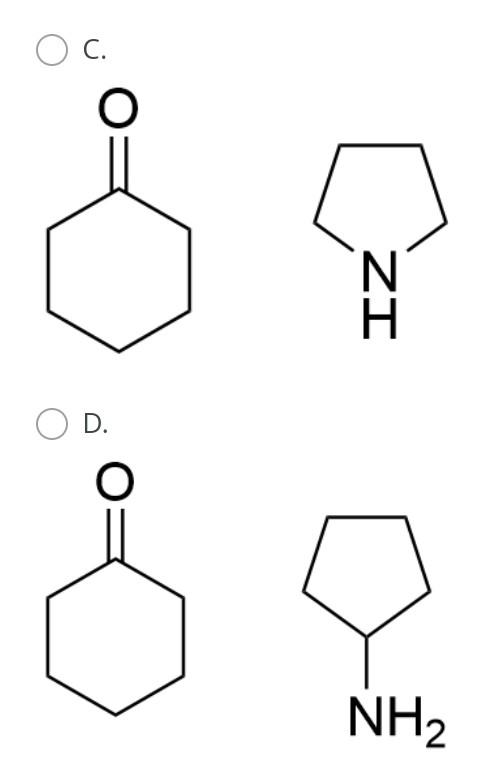
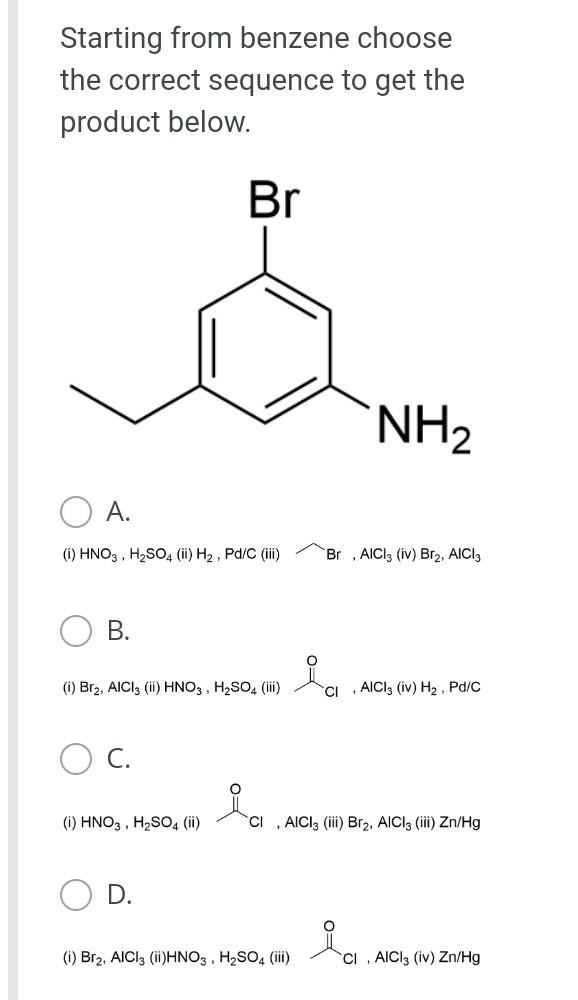
The general orbital interaction diagram (valence electrons only) for the formation of a homonuclear diatomic molecule X2 is given below. Use this to predict which one of the following species will have the highest bond dissociation energy: 9."(20 Energy T*(2) * 2p 3, (2p) (20) 2p 2p (20) (23) 2s 2s (25) X X A. [F2]2+ B. [F2] C. F2 D. [F2]* Which molecule (A to D) has an absorption near 2150 cm-?? A B CECH C D OH A. A B. B O C. D. D Provide the IUPAC name for [Mn(OH)(OH2). Cl2: A. pentaaquahydroxidomanganate(III) chloride B. hydroxidopentaaquamanganese(III) chloride Oc. pentaaquahydroxidomanganese(III) chloride D. pentaaquahydroxidomanganese chloride Choose the correct starting materials to produce: N N A. OH O ZI O B. NH2 O O c. O O ZI OD. NH2 Starting from benzene choose the correct sequence to get the product below. Br NH2 A. (i) HNO3, H2SO4 (ii) H2, Pd/C (iii) Br , AICI(iv) Br2, AICI o B. la (0) Br2, AICI; (ii) HNO3, H2SO4 (ii) CI AICI: (iv) H2, Pd/C C. () HNO3, H2SO4 (ii) CI, AICI; (ii) Bra. AlCl3 (iii) Zn/Hg D. id (1) Bra, AICI: (i)HNO3 , H2SO4 (iii) CI, AICI; (iv) Zn/HgStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


