Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Thank you very much for answering the parts of this long question, will definitely thumbs up:) Figure 1: The Five types of physisorption isotherms for

Thank you very much for answering the parts of this long question, will definitely thumbs up:)
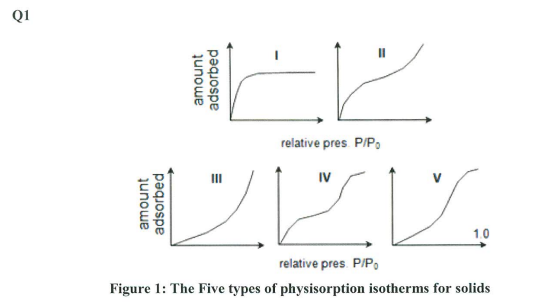
Figure 1: The Five types of physisorption isotherms for solids (a) Figure 1 shows the five generic types of physisorption isotherms found over all solids. Describe any four of these isotherms with respect to the porosity of the solid under study. (4 marks) (b) What are the basic assumptions of the BET isotherm? (3 marks) (c) Explain why a BET isotherm is used as opposed to a Langmuir isotherm (i.e., what are the limitations of the Langmuir isotherm)? (4 marks) (d) The data in Table 1, relates to the absorption of N2 on nano- TiO2 at 75K. Confirm that this data fits a BET isotherm in the range of pressures given in the table and determine values for Vm, and c. Useful Equations V/1P/P0/P/P0=cVm1+cVmc1(P/P0)(P/P0)Vm=m+b1c=bm+1As=AmNm=AmVT,PVm6.0221023 gas volume is 22.4L/mol at STP, Avogadro 's number 6.0221023mole1.) (10 marks) (e) Briefly explain what the values of Vm, and c, determined above represent. Use these values and the details supplied above to determine the surface area of the nano- TiO2. (4 marks) Figure 1: The Five types of physisorption isotherms for solids (a) Figure 1 shows the five generic types of physisorption isotherms found over all solids. Describe any four of these isotherms with respect to the porosity of the solid under study. (4 marks) (b) What are the basic assumptions of the BET isotherm? (3 marks) (c) Explain why a BET isotherm is used as opposed to a Langmuir isotherm (i.e., what are the limitations of the Langmuir isotherm)? (4 marks) (d) The data in Table 1, relates to the absorption of N2 on nano- TiO2 at 75K. Confirm that this data fits a BET isotherm in the range of pressures given in the table and determine values for Vm, and c. Useful Equations V/1P/P0/P/P0=cVm1+cVmc1(P/P0)(P/P0)Vm=m+b1c=bm+1As=AmNm=AmVT,PVm6.0221023 gas volume is 22.4L/mol at STP, Avogadro 's number 6.0221023mole1.) (10 marks) (e) Briefly explain what the values of Vm, and c, determined above represent. Use these values and the details supplied above to determine the surface area of the nano- TiO2. (4 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


