Question
The 2nd order dimerization reaction 2A B (rA = -kCA 2 ) takes place in the liquid phase, being k = 0.01 dm3/(molmin) at the
The 2nd order dimerization reaction 2A B (rA = -kCA2) takes place in the liquid phase, being k = 0.01 dm3/(molmin) at the reaction temperature. The food is pure A with a concentration of 8 mol/L. The theoretical volume of the reactor is 1000 L and the flow rate feed for dimming is 25 L/min
In order to know the behavior of the reactor, which does not behave ideally, it has been developed a test with impulse tracer with a flow rate of 25 L/min, whose results are shown in the following table:
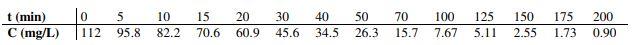
It is desired to know the limits between which the conversion can vary depending on the degree of micromix.
200 t (min) C (mg/L) 5 95.8 10 82.2 15 70.6 als 20 60.9 30 45.6 40 34.5 50 26.3 70 15.7 100 7.67 125 5.11 150 2.55 175 1.73 o A 112 0.90Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


