Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The absorbance for a dye sample of unknown concentration is measured and the corrected absorbance is 0.622. Use the calibration curve in the above figure
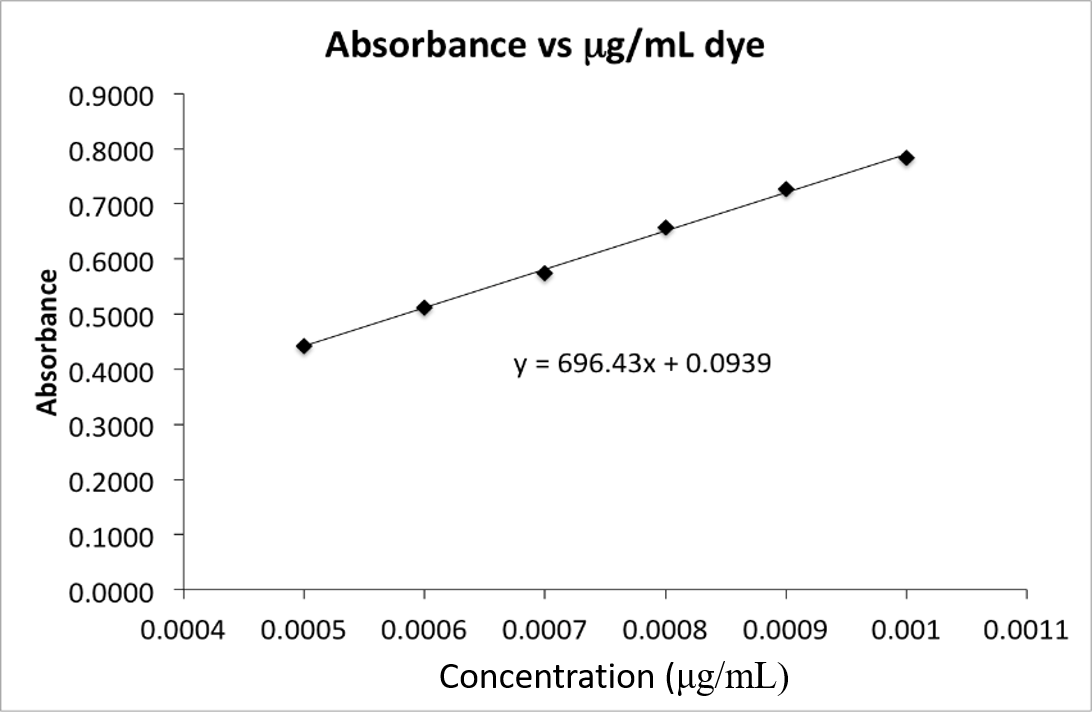
The absorbance for a dye sample of unknown concentration is measured and the corrected absorbance is 0.622. Use the calibration curve in the above figure to calculate the concentration of the dye sample.
| A. | 5.80 x 10-4 g/mL | |
| B. | 6.88 x 10-4 g/mL | |
| C. | 7.58 x 10-4 g/mL | |
| D. | 0.0939 g/mL | |
| E. | 9.46 x 10-4 g/mL |
Absorbance vs ug/mL dye 0.9000 0.8000 0.7000 0.6000 0.5000 Absorbance 0.4000 y = 696.43x + 0.0939 0.3000 0.2000 0.1000 0.0000 0.0004 0.0005 0.0006 0.0007 0.0008 0.0009 0.001 0.0011 Concentration (ug/mL)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


