Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The acetic acid (HOAC) content of vinegar is determined by titrating with a standard solution of sodium hydroxide to a phenolphthalein end point. (a)
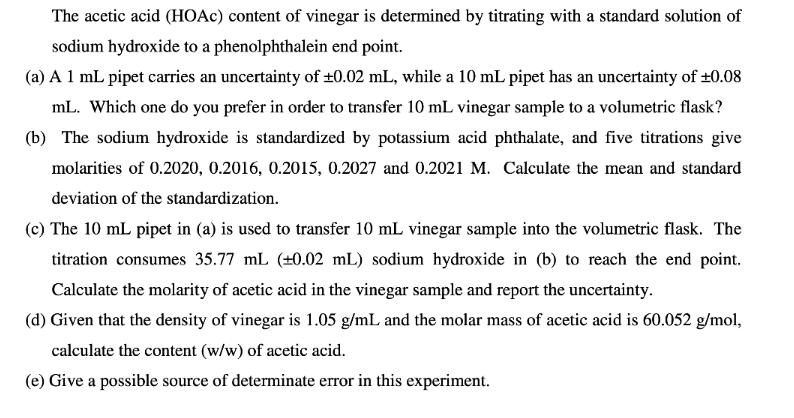
The acetic acid (HOAC) content of vinegar is determined by titrating with a standard solution of sodium hydroxide to a phenolphthalein end point. (a) A 1 mL pipet carries an uncertainty of 0.02 mL, while a 10 mL pipet has an uncertainty of +0.08 mL. Which one do you prefer in order to transfer 10 mL vinegar sample to a volumetric flask? (b) The sodium hydroxide is standardized by potassium acid phthalate, and five titrations give molarities of 0.2020, 0.2016, 0.2015, 0.2027 and 0.2021 M. Calculate the mean and standard deviation of the standardization. (c) The 10 mL pipet in (a) is used to transfer 10 mL vinegar sample into the volumetric flask. The titration consumes 35.77 mL (+0.02 mL) sodium hydroxide in (b) to reach the end point. Calculate the molarity of acetic acid in the vinegar sample and report the uncertainty. (d) Given that the density of vinegar is 1.05 g/mL and the molar mass of acetic acid is 60.052 g/mol, calculate the content (w/w) of acetic acid. (e) Give a possible source of determinate error in this experiment.
Step by Step Solution
★★★★★
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
a 10ml pipette is the correct one as the error is less than using 1ml ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


