Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The actual formula for hydrated copper chloride is CuCl 2 .2H 2 O. Your sample: 0.0116 mol H 2 0 0.0058 mol Cu 0.01511 mol
The actual formula for hydrated copper chloride is CuCl2.2H2O.
Your sample:
0.0116 mol H20
0.0058 mol Cu
0.01511 mol Cl

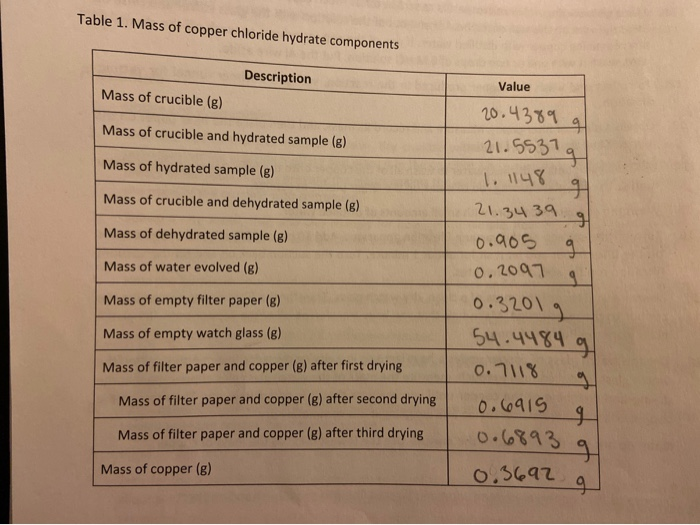
5. The actual formula for hydrated copper chloride is CuCl-2HO. a. Based on this formula, how many grams of water were present in your sample of hydrated copper chloride? b. Calculate the percent error of your measured mass of water compared to the theoretical mass of water in the sample. C. % Error: = lexperimental-theoretical theoretical x 100% Equation 1 Based on this formula, how many grams of copper were present in your sample of hydrated copper chloride? d. Calculate the percent error of your measured mass of copper compared to the actual mass of copper in the sample.
Step by Step Solution
★★★★★
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


