Question
The ammonia decomposition is carried out at 856C in an intermittent reactor at constant density, obtaining the following data: Time (s) Total pressure (mmhg)
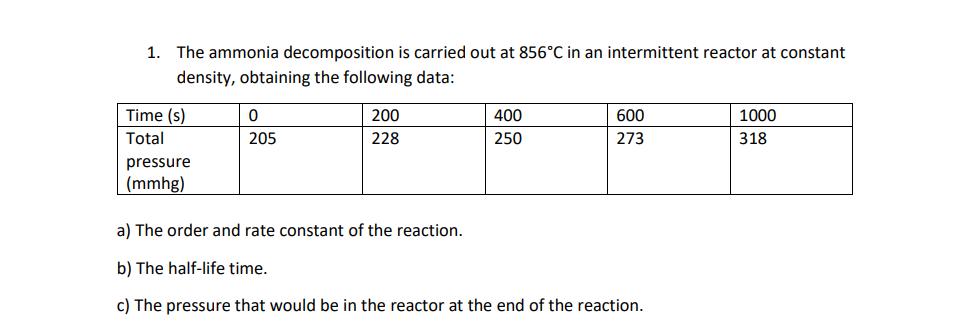
The ammonia decomposition is carried out at 856C in an intermittent reactor at constant density, obtaining the following data: Time (s) Total pressure (mmhg) 0 205 200 228 400 250 600 273 a) The order and rate constant of the reaction. b) The half-life time. c) The pressure that would be in the reactor at the end of the reaction. 1000 318
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Momentum Heat And Mass Transfer
Authors: James Welty, Gregory L. Rorrer, David G. Foster
6th Edition
1118947460, 978-1118947463
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



