Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In
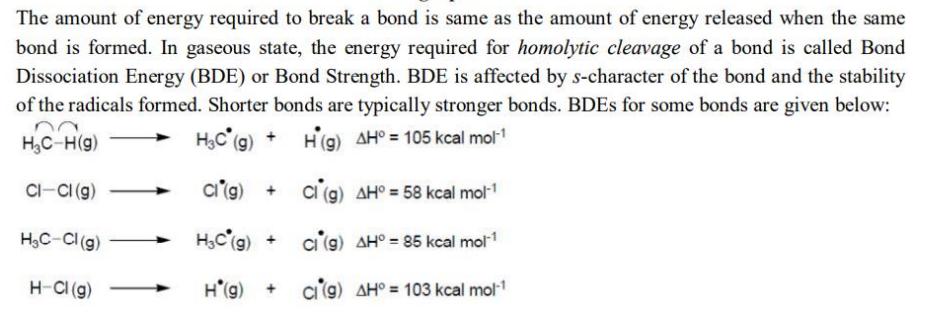
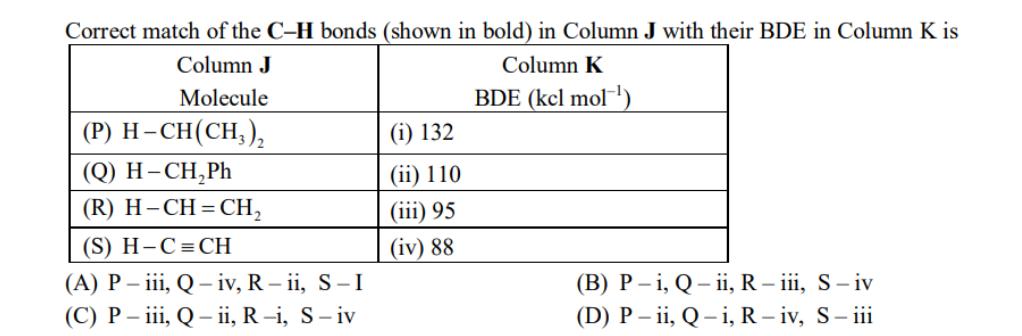
The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the energy required for homolytic cleavage of a bond is called Bond Dissociation Energy (BDE) or Bond Strength. BDE is affected by s-character of the bond and the stability of the radicals formed. Shorter bonds are typically stronger bonds. BDEs for some bonds are given below: HC-H(g) HC (g) H(g) AH = 105 kcal mol-1 CI-CI (g) HC-Cl (g) H-Cl (g) Ci (g) + HC (g) + H*(g) + Cl (g) AH = 58 kcal mol- Ci (9) AH = 85 kcal mol- ci (g) AH = 103 kcal mol- Correct match of the C-H bonds (shown in bold) in Column J with their BDE in Column K is Column J Column K Molecule BDE (kel mol-) (P) H-CH(CH3) (Q) H-CHPh (R) H-CH=CH (S) H-C=CH (A) P-iii, Q-iv, R-ii, S-I (C) P-iii, Q-ii, R-i, S-iv (1) 132 (ii) 110 (iii) 95 (iv) 88 (B) P-i, Q-ii, R - iii, S-iv (D) P-ii, Q-i, R-iv, S-iii
Step by Step Solution
★★★★★
3.33 Rating (141 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


