Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the answer is 1.13 x 10^-6 i just dont know how to get that. can someone please help me? As required in the previous quiz
the answer is 1.13 x 10^-6 i just dont know how to get that. can someone please help me? 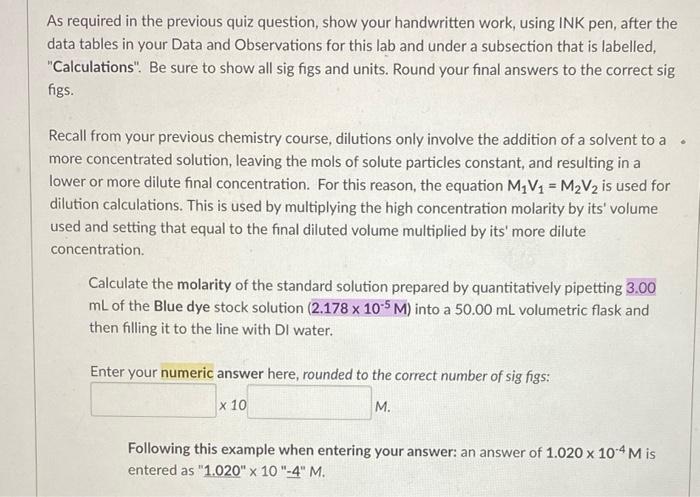
As required in the previous quiz question, show your handwritten work, using INK pen, after the data tables in your Data and Observations for this lab and under a subsection that is labelled, "Calculations". Be sure to show all sig figs and units. Round your final answers to the correct sig figs. Recall from your previous chemistry course, dilutions only involve the addition of a solvent to a more concentrated solution, leaving the mols of solute particles constant, and resulting in a lower or more dilute final concentration. For this reason, the equation M V2 = M2V2 is used for dilution calculations. This is used by multiplying the high concentration molarity by its' volume used and setting that equal to the final diluted volume multiplied by its' more dilute concentration. Calculate the molarity of the standard solution prepared by quantitatively pipetting 3.00 mL of the Blue dye stock solution (2.178 x 10-5M) into a 50.00 mL volumetric flask and then filling it to the line with Di water. Enter your numeric answer here, rounded to the correct number of sig figs: x 10 M. Following this example when entering your answer: an answer of 1.020 x 10-4 M is entered as "1.020" x 10"-4" M 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


