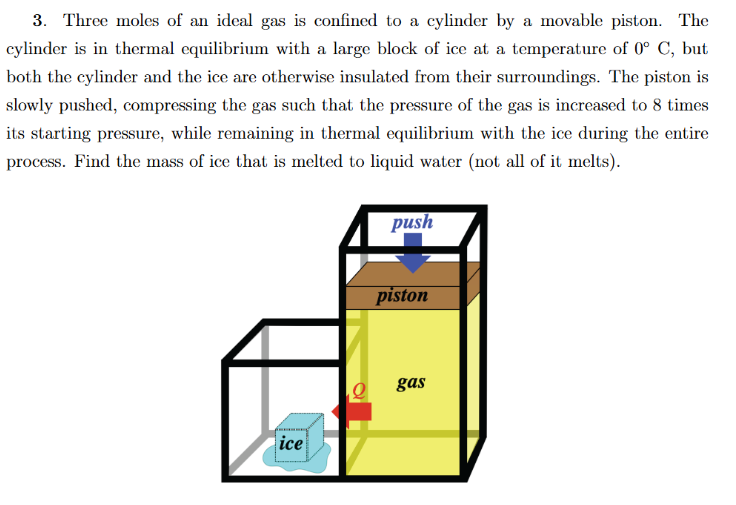
Question: THE ANSWER IS 4 2 g SHOW ALL STEPS AND GET RIGHT ANSWER AND I WILL THUMBS UP THANK YOU. Three moles of an ideal
THE ANSWER IS g SHOW ALL STEPS AND GET RIGHT ANSWER AND I WILL THUMBS UP THANK YOU. Three moles of an ideal gas is confined to a cylinder by a movable piston. The
cylinder is in thermal equilibrium with a large block of ice at a temperature of @C but
both the cylinder and the ice are otherwise insulated from their surroundings. The piston is
slowly pushed, compressing the gas such that the pressure of the gas is increased to times
its starting pressure, while remaining in thermal equilibrium with the ice during the entire
process. Find the mass of ice that is melted to liquid water not all of it melts
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


