Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The answer is d, but I'm unsure as how to get it. 5. In the case of anionic polymerization using organometallic initiator, Abby was able
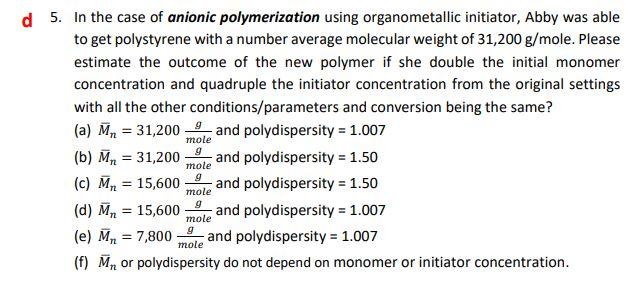
The answer is d, but I'm unsure as how to get it.
5. In the case of anionic polymerization using organometallic initiator, Abby was able to get polystyrene with a number average molecular weight of 31,200g/ mole. Please estimate the outcome of the new polymer if she double the initial monomer concentration and quadruple the initiator concentration from the original settings with all the other conditions/parameters and conversion being the same? (a) Mn=31,200moleg and polydispersity =1.007 (b) Mn=31,200moleg and polydispersity =1.50 (c) Mn=15,600moleg and polydispersity =1.50 (d) Mn=15,600moleg and polydispersity =1.007 (e) Mn=7,800moleg and polydispersity =1.007 (f) Mn or polydispersity do not depend on monomer or initiator concentrationStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


