Question
The aqueous nitric acid solution is fed at a flow rate of 20000 kg/h and contains 50% nitric acid by mass . After the fresh
The aqueous nitric acid solution is fed at a flow rate of 20000 kg/h and contains 50% nitric acid by mass. After the fresh acid feed stream is mixed with two return streams coming from different points of the process, it is heated by giving Qh energy and fed to the reactor. Here it enters the following reaction with ammonia gas fed at 108C and 4.5 bar:
NH3(g) + HNO3(aq) NH4NO3(aq)
Ammonia fed to the reactor is excessive compared to nitric acid and the reaction takes place in 100% yield. The reactor outlet stream at 210 C and in the form of a gas-liquid mixture passes into a cyclone separation unit. Here, the liquid NH4NO3 drops hurled by the effect of centrifugal force adhere to the cyclone wall and descend to the cyclone floor, where it comes into contact with hot air. The hot air evaporates all the water in the liquid and some ammonium nitrate. The remaining ammonium nitrate leaves the cyclone in melt form. Before being fed into the cyclone, the air is heated from 25 to 160 with the help of a heat exchanger where the cyclone outlet is used as a hot stream.
The molten ammonium nitrate leaving the cyclone at 155 C is cooled in a cooling unit and completely solidified. Solid ammonium nitrate is then ground and sieved. Fine powdered parts are separated and returned to the process; The remaining product is packed and sent to the shipping department. Powdered ammonium nitrate is 75% by mass of the total ammonium nitrate fed to the grinder.
The mixture leaving the cyclone at 185 C contains hot air, water, excess ammonia and ammonium nitrate. The amount of ammonium nitrate in this stream is 25% by mass of the total ammonium nitrate leaving the reactor. The current is cooled to Ta temperature by passing through a heat exchanger and fed to the Condenser I unit.
NH3 and H2O liquid-gas phases at the exit of the condenser I are in equilibrium at 115 . The partial pressure of NH3 in the gas phase (the current sent to Condenser II) is 2048 mm-Hg, the partial pressure of H2O is 576 mm-Hg, and the total pressure is 5080 mm-Hg. A, B, C constants required to use the Antoine equation are given in the attached table. In addition, the mass ratio (m17/m14) of the water in the gas phase to the water in the liquid phase at the Condenser I output is given as 2:1. The gas phase leaving Condenser I is fed to Condenser II; Here, all the air is separated and released to the atmosphere, while water and NH3 are taken as liquids.
| A | B | C | |
| NH3 | 7.55466 | 1002.711 | 247.885 |
| H2O | 7.96681 | 1668.210 | 228.000 |
| Ax103 | Bx105 | Cx108 | Dx1012 | |
| NH3 | 35.15 | 2.954 | 0.4421 | -6.686 |
| H2O | 33.46 | 0.6880 | 0.7064 | -3.593 |
| HNO3 | 110.0 | - | - | - |
| NH4NO3 | 215.9 | - | - | - |
| Air | 28.94 | 0.4147 | 0.3191 | -1.965 |
NH3(g) + HNO3(aq) NH4NO3(aq) "" = 145.1'(/)*+ (25 , 1 atm)
A, B, C and D constants in the Cp equation of NH3, H2O, HNO3, NH4NO3 and air are given in the attached table. Write a function that calculates enthalpy using the following formula, using Python.
The function should accept the relevant constants and the temperatures T1 and T2 as inputs. Calculate the required enthalpy values by running the code you wrote.
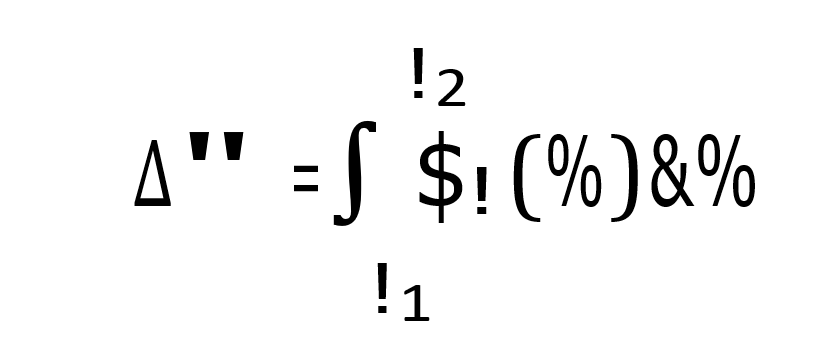
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


