Question: The arrow at A should be pointing upwards In this question, you will calculate the enthaipies of solution and determine the relative solubility of unknown

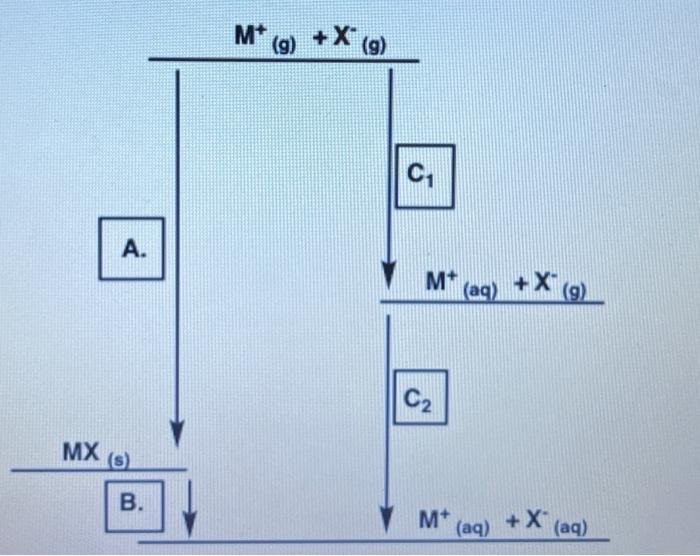
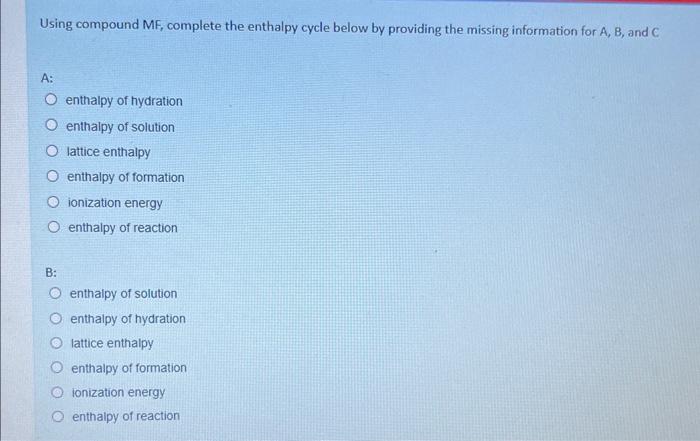
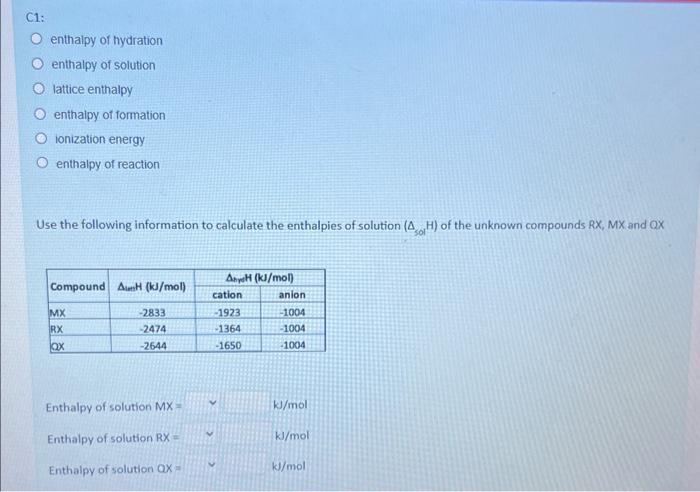
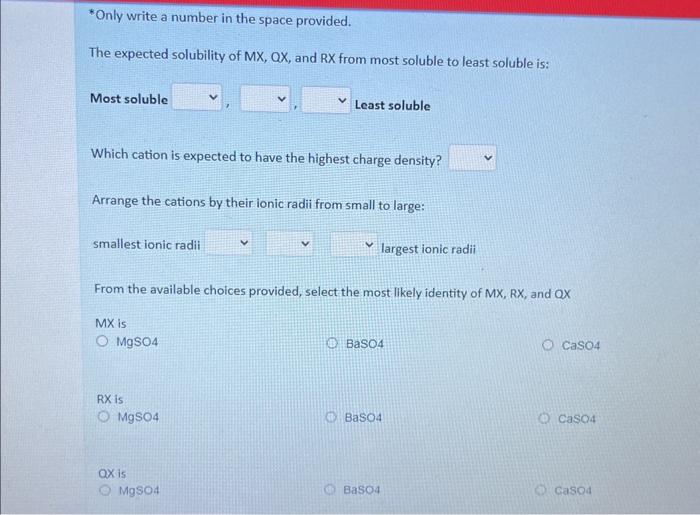
In this question, you will calculate the enthaipies of solution and determine the relative solubility of unknown Group 1 fluorides RF, MF, and QF and use this information to determine the identity of elements R, M and Q. Using MF, complete the enthalpy cycle below by providing the missing information for A, B, and C Using compound MF, complete the enthalpy cycle below by providing the missing information for A, B, and C. A: enthalpy of hydration enthalpy of solution lattice enthalpy enthalpy of formation ionization energy enthalpy of reaction B: enthalpy of solution enthalpy of hydration lattice enthalpy enthalpy of formation Ionization energy enthalpy of reaction enthalpy of hydration enthalpy of solution lattice enthalpy enthalpy of formation ionization energy enthalpy of reaction Use the following information to calculate the enthalpies of solution (solH) of the unknown compounds RX,MX and QX Enthalpy of solution MX= kJ/mol Enthalpy of solution RX= kJ/mol Enthalpy of solution ax= kJ/mol *Only write a number in the space provided. The expected solubility of MX,QX, and RX from most soluble to least soluble is: Most soluble Least soluble Which cation is expected to have the highest charge density? Arrange the cations by their ionic radii from small to large: smallest ionic radii largest ionic radii From the available choices provided, select the most likely identity of MX,RX, and QX MX is MgSO4 BaSO4 CaSO4 RX is MgSO4 Baso4 CaSO4 QX is MgSO4 Baso4 Casod
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


