Question
The atomic orbital sets of similar energy are grouped into a series of seven shells, which is equal to the number of periods in the
The atomic orbital sets of similar energy are grouped into a series of seven shells, which is equal to the number of periods in the periodic table. Complete the energy-level diagram by filling the number of electrons in each orbital set within a shell, and also give the total number of electrons in each shell. The first shell has been filled in for you as an example.
Drag the appropriate labels to their respective targets.
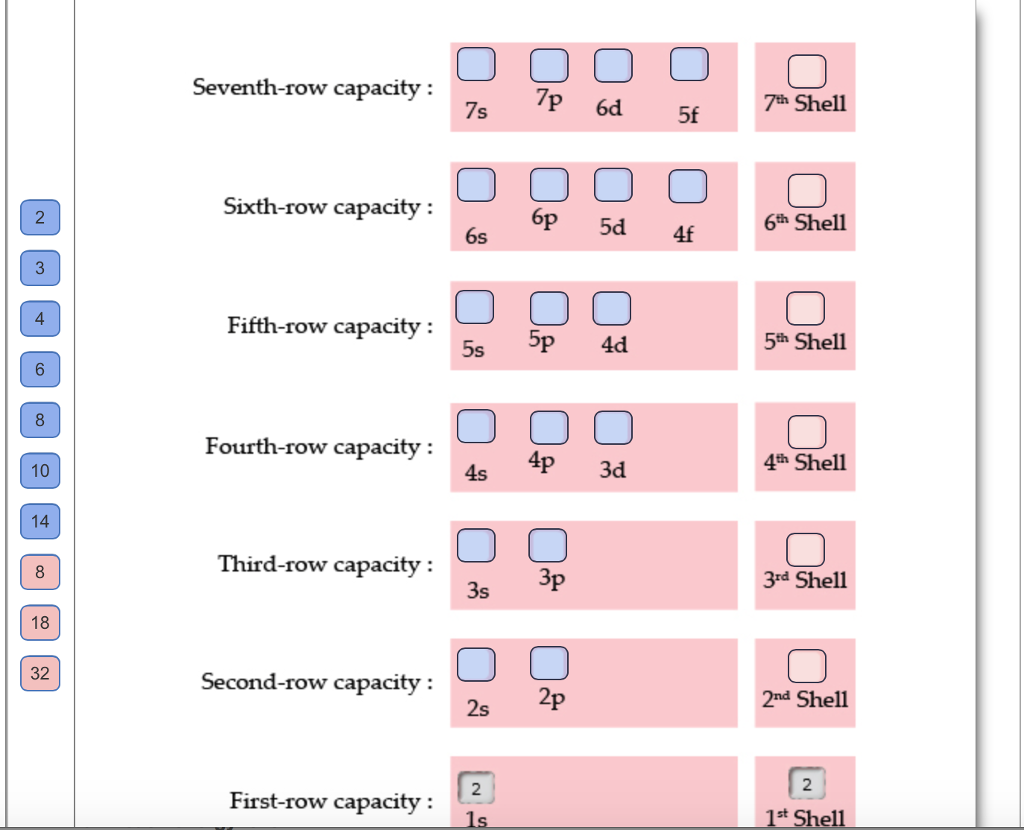
3 4 8 10 14 8 18 32 Seventh-row capacity: Sixth-row capacity: Fifth-row capacity: Fourth-row capacity: Third-row capacity: Second-row capacity: First-row capacity: 7p 7s 6s 5s 5p 4s 3s 2s 1s 5d 4p 3p 6d 2p 4d 3d 5f 4f 7th Shell 6th Shell 5th Shell 4th Shell 3rd Shell 2nd Shell 2 1st Shell
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



