Question
The Beer?s Law plot is one of the most useful for a quantitative analytical chemist. Examine the following Beer?s Law plot. a. What chemical and
The Beer?s Law plot is one of the most useful for a quantitative analytical chemist. Examine the following Beer?s Law plot.
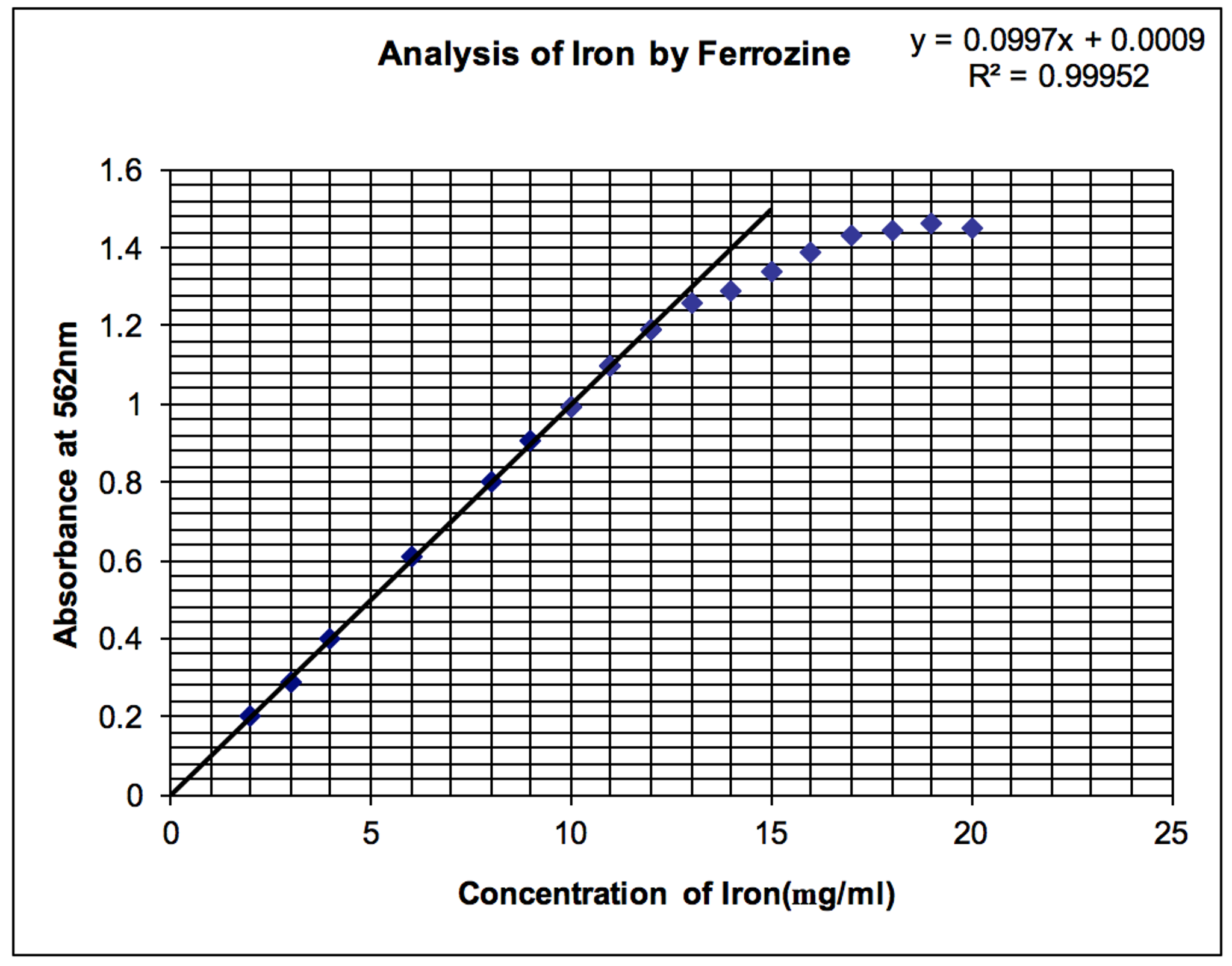
a. What chemical and physical reasons (there are both) cause the calibration curve to flatten at high concentrations and absorbances?
b. Given the equation of the line, what is the concentration of the sample if the absorbance is 0.743 absorbance units?
c. The sample was prepared by taking a 10 mL sample from the stock solution and diluting it to 100mL. What is the concentration of the stock solution based on your answer from part b?
d. What is the molar absorptivity for the iron complex (assume that the Fe: complex ratio is 1:1) at this wavelength? You may assume that the path length is 1.00 cm.
Analysis of Iron by Ferrozine y = 0.0997x + 0.0009 R2 = 0.99952 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 10 15 20 25 Concentration of Iron(mg/ml) Absorbance at 562nm
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
At higher concentrations the molecules can associated di...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


