Question
The behaviour of a certain gas is described by the van der Waals equation 2 P = 0.02T V-0.007 V2 where P is pressure,
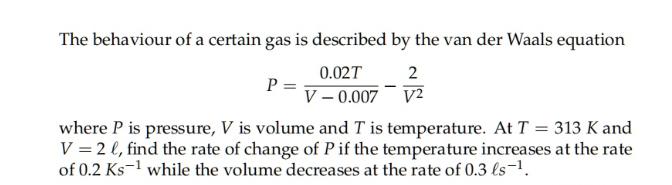
The behaviour of a certain gas is described by the van der Waals equation 2 P = 0.02T V-0.007 V2 where P is pressure, V is volume and T is temperature. At T = 313 K and V = 2 l, find the rate of change of P if the temperature increases at the rate of 0.2 Ks while the volume decreases at the rate of 0.3 ls-.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Given 20 2T Using chain rule de ap 2P ar P ap av Given dp 002 T ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Numerical Methods With Chemical Engineering Applications
Authors: Kevin D. Dorfman, Prodromos Daoutidis
1st Edition
1107135117, 978-1107135116
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



