Question
The figure below shows a self-cooling beverage can. The can is equipped with an outer jacket containing sodium carbonate (Na 2 CO 3 ), which
The figure below shows a “self-cooling” beverage can.
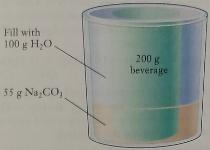
The can is equipped with an outer jacket containing sodium carbonate (Na2CO3), which dissolves in water rapidly and endothermically.
Na2CO3(s) → 2 Na+ (aq) + CO32−(aq) ΔH° = 67.7 kJ
The user adds water to the outer jacket, and the heat absorbed in the chemical reaction chills the drink. The can contains 200 g of drink, the jacket contains 55 g Na2CO3, and 100 g of water is to be added. If the initial temperatures of the can and the water are 32°C on a summer day, what is the coldest temperature that the drink can reach? The can itself has a heat capacity of 40 J/°C. Assume that the Na2CO3 solution and drink both have the same heat capacity as pure water, 4.184 J g−1 °C−1.
Fill with 100 g H;O 200 g beversge 55 g Na CO,
Step by Step Solution
3.32 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Mass of Na2CO3 55 g Moles of Na2CO3 mass molar mass 55 g 10...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


