Answered step by step
Verified Expert Solution
Question
1 Approved Answer
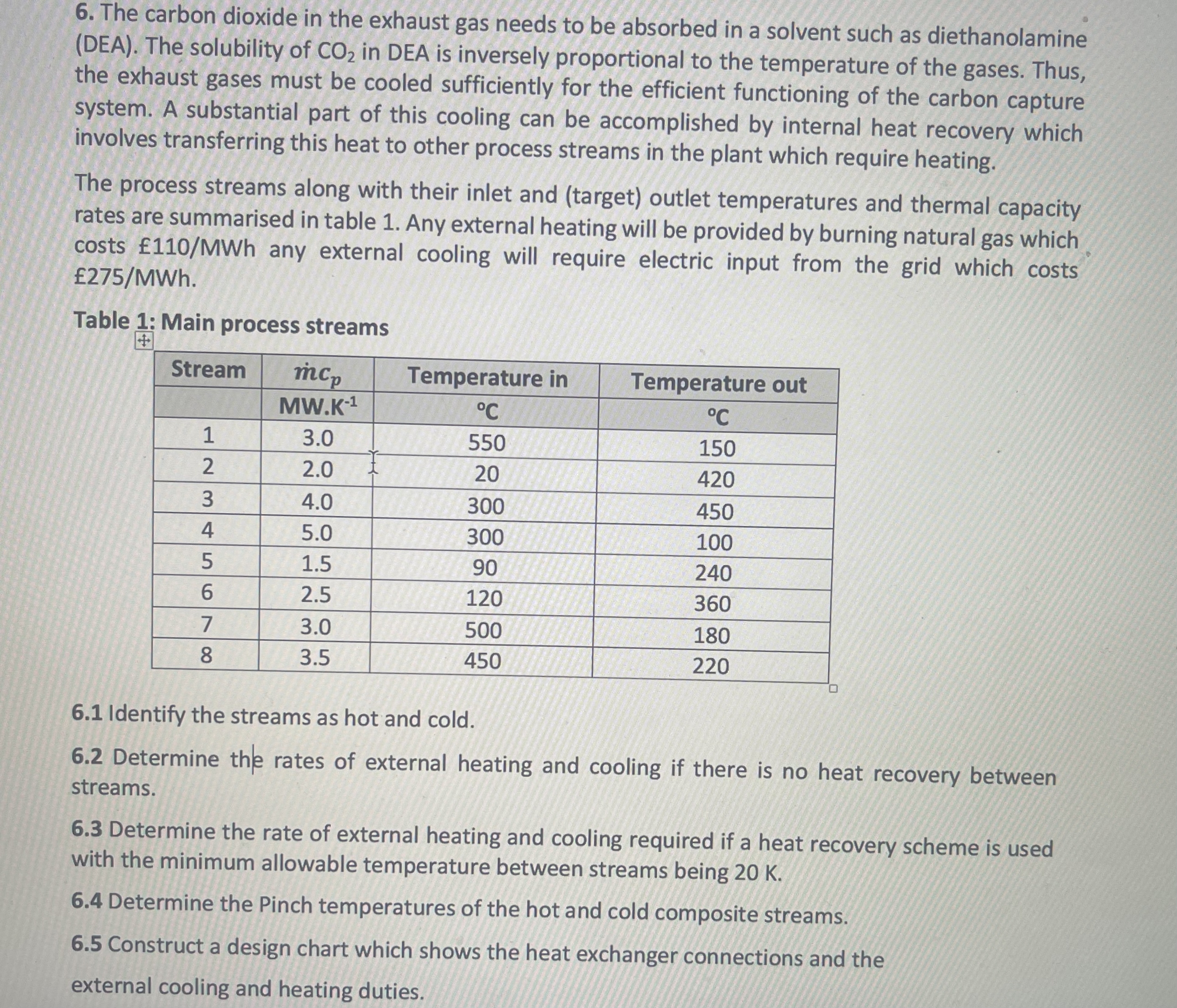
The carbon dioxide in the exhaust gas needs to be absorbed in a solvent such as diethanolamine ( DEA ) . The solubility of C
The carbon dioxide in the exhaust gas needs to be absorbed in a solvent such as diethanolamine DEA The solubility of in DEA is inversely proportional to the temperature of the gases. Thus, the exhaust gases must be cooled sufficiently for the efficient functioning of the carbon capture system. A substantial part of this cooling can be accomplished by internal heat recovery which involves transferring this heat to other process streams in the plant which require heating.
The process streams along with their inlet and target outlet temperatures and thermal capacity rates are summarised in table Any external heating will be provided by burning natural gas which costs any external cooling will require electric input from the grid which costs
Table : Main process streams
tableStreamTemperature inTemperature out
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


