Answered step by step
Verified Expert Solution
Question
1 Approved Answer
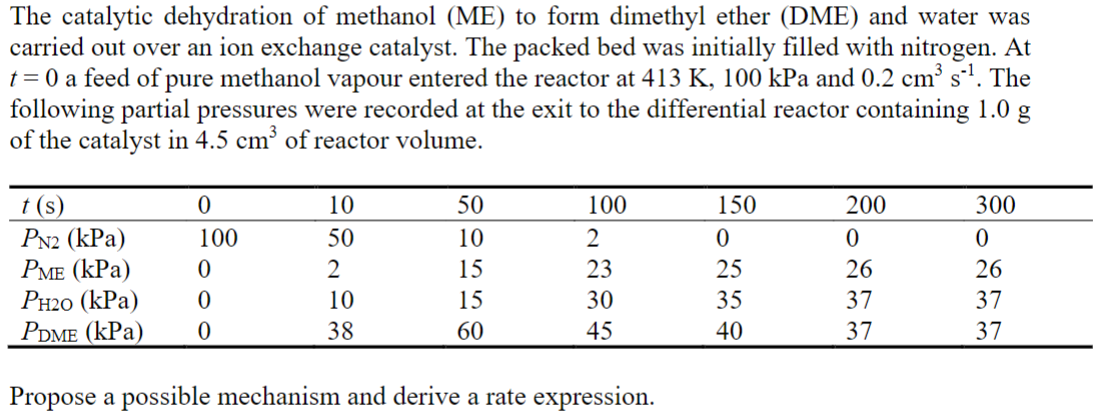
The catalytic dehydration of methanol ( ME ) to form dimethyl ether ( DME ) and water was carried out over an ion exchange catalyst.
The catalytic dehydration of methanol ME to form dimethyl ether DME and water was
carried out over an ion exchange catalyst. The packed bed was initially filled with nitrogen. At
a feed of pure methanol vapour entered the reactor at kPa and The
following partial pressures were recorded at the exit to the differential reactor containing
of the catalyst in of reactor volume.
Propose a possible mechanism and derive a rate expression.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


